Physicochemical Properties
| Molecular Formula | C32H31N3O8 |
| Molecular Weight | 585.60 |
| Exact Mass | 585.211 |
| Elemental Analysis | C, 65.63; H, 5.34; N, 7.18; O, 21.86 |
| CAS # | 1473403-87-0 |
| PubChem CID | 146673122 |
| Appearance | White to yellow solid powder |
| LogP | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Heavy Atom Count | 43 |
| Complexity | 1300 |
| Defined Atom Stereocenter Count | 1 |
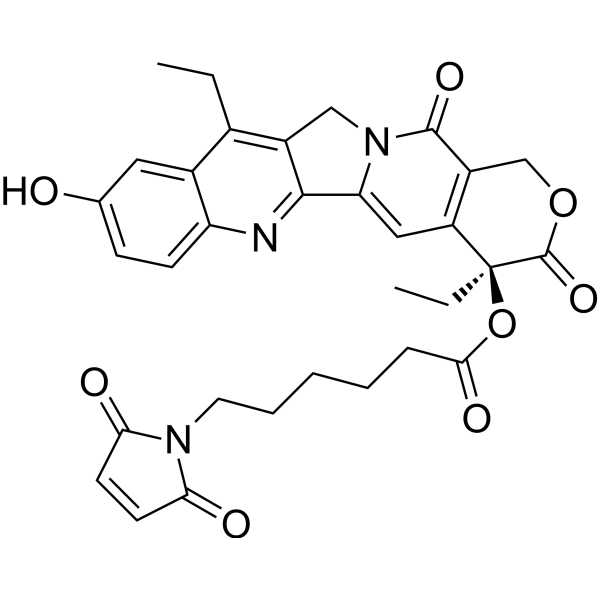
| SMILES | O=C1C=CC(=O)N1CCCCCC(O[C@@]1(C(OCC2C(N3CC4=C(CC)C5=CC(=CC=C5N=C4C3=CC=21)O)=O)=O)CC)=O |
| InChi Key | ACRMADURFCYBAU-YTTGMZPUSA-N |
| InChi Code | InChI=1S/C32H31N3O8/c1-3-19-20-14-18(36)9-10-24(20)33-29-21(19)16-35-25(29)15-23-22(30(35)40)17-42-31(41)32(23,4-2)43-28(39)8-6-5-7-13-34-26(37)11-12-27(34)38/h9-12,14-15,36H,3-8,13,16-17H2,1-2H3/t32-/m0/s1 |
| Chemical Name | [(19S)-10,19-diethyl-7-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-19-yl] 6-(2,5-dioxopyrrol-1-yl)hexanoate |
| Synonyms | MC-SN38; 1473403-87-0; AKOS040756843; [(19S)-10,19-diethyl-7-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-19-yl] 6-(2,5-dioxopyrrol-1-yl)hexanoate; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Camptothecins/DNA Topoisomerase I; Drug-linker conjugate for ADC |
| ln Vitro | It is known that 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11), a semisynthesized derivative of camptothecin (CPT), has a potent antitumor activity in vivo, but 7-ethyl-10-hydroxycamptothecin (SN-38), a metabolite of CPT-11, shows much stronger cytotoxicity in vitro than CPT-11. In this study, we demonstrated that the relaxation of SV40 DNA plasmids by type I DNA topoisomerase prepared from P388 murine leukemia cells was inhibited by 50% by SN-38 at approximately 1 microM, although CPT-11 at 1 mM slightly inhibited the relaxation. SN-38 and CPT showed strong, time-dependent inhibitory activity against DNA synthesis of P388 cells. However, CPT-11 weakly inhibited DNA synthesis independently of time with coincident inhibition of the total thymidine uptake by the cells. By alkaline and neutral elution assays, it was demonstrated that SN-38 caused much more frequent DNA single-strand breaks in P388 cells than did CPT-11. The same content of SN-38 and a similar frequency of single-strand breaks were detected in the cells treated with SN-38 at 0.1 microM or with CPT-11 at 100 microM. Therefore, single-strand breaks by CPT-11 seem to be due to SN-38 produced from CPT-11 in cells. These results indicate that CPT-11 itself possesses a marginal antiproliferative effect but that SN-38 plays an essential role in the mechanism of action of CPT-11[2]. |
| References |
[1]. Characterization of DNA topoisomerase I in three SN-38 resistant human colon cancer cell lines reveals a newpair of resistance-associated mutations. J Exp Clin Cancer Res. 2016 Mar 31;35:56. [2]. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51(16):4187-4191. |
| Additional Infomation | DNA topoisomerase I (Top1) is a DNA unwinding protein and the specific target of the camptothecin class of chemotherapeutic drugs. One of these, irinotecan, acting through its active metabolite SN-38, is used in the treatment of metastatic colorectal cancer. However, resistance to irinotecan represents a major clinical problem. Since molecular alterations in Top1 may result in resistance to irinotecan, we characterized Top1 in three human colon cancer cell lines with acquired resistance to SN-38. Methods: Three SN-38 resistant (20-67 fold increased resistance) cell lines were generated and compared to wild-type parental cells with regards to: TOP1 gene copy number and gene sequence, Top1 expression (mRNA and protein), Top1 enzymatic activity in the absence and presence of drug, and Top1-DNA cleavage complexes in drug treated cells. TOP1 mutations were validated by PCR using mutant specific primers. Furthermore, cross-resistance to two indenoisoquinoline Top1-targeting drugs (NSC 725776 and NSC 743400) and two Top2-targeting drugs (epirubicin and etoposide) was investigated. Results: Two of three SN-38 resistant cell lines carried TOP1 gene copy number aberrations: A TOP1 gene copy gain and a loss of chromosome 20, respectively. One resistant cell line harbored a pair of yet unreported TOP1 mutations (R364K and G717R) in close proximity to the drug binding site. Mutant TOP1 was expressed at a markedly higher level than wild-type TOP1. None or very small reductions were observed in Top1 expression or Top1 activity in the absence of drug. In all three SN-38 resistant cell lines Top1 activity was maintained in the presence of high concentrations of SN-38. None or only partial cross-resistance were observed for etoposide and epirubicin, respectively. SN-38 resistant cells with wild-type TOP1 remained sensitive to NSC 743400, while cells with mutant TOP1 was fully cross-resistant to both indenoisoquinolines. Top1-DNA cleavage complex formation following drug treatment supported the other findings. Conclusions: This study adds to the growing knowledge about resistance mechanisms for Top1-targeting chemotherapeutic drugs. Importantly, two yet unreported TOP1 mutations were identified, and it was underlined that cross-resistance to the new indenoisoquinoline drugs depends on the specific underlying molecular mechanism of resistance to SN-38.[1] |
Solubility Data
| Solubility (In Vitro) | DMSO : 140 mg/mL (239.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5.75 mg/mL (9.82 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 57.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 5.75 mg/mL (9.82 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 57.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7077 mL | 8.5383 mL | 17.0765 mL | |
| 5 mM | 0.3415 mL | 1.7077 mL | 3.4153 mL | |
| 10 mM | 0.1708 mL | 0.8538 mL | 1.7077 mL |