Lixisenatide acetate (AVE-0010; ZP-10A; Adlyxin; Lyxumia; ZP10A), the acetate salt of Lixisenatide, is a potent and short-acting agonist of the glucagon-like peptide-1 receptor (GLP-1R) approved in 2016 for the treatment of type 2 diabetes mellitus (T2DM). It activates GLP-1R with an IC50 value of 1.4 nM for the human GLP-1 receptor in in vitro receptor binding studies.
Physicochemical Properties
| Molecular Formula | C215H347N61O65S |
| Molecular Weight | 4858.4904282093 |
| Exact Mass | 4857.551 |
| Elemental Analysis | C, 53.15; H, 7.20; N, 17.59; O, 21.40; S, 0.66 |
| CAS # | 1997361-87-1 |
| Related CAS # | Lixisenatide; 320367-13-3 |
| PubChem CID | 16139342 |
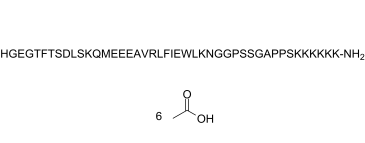
| Sequence | H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Ser-Lys-Lys-Lys-Lys-Lys-Lys-NH2; L-histidyl-glycyl-L-alpha-glutamyl-glycyl-L-threonyl-L-phenylalanyl-L-threonyl-L-seryl-L-alpha-aspartyl-L-leucyl-L-seryl-L-lysyl-L-glutaminyl-L-methionyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alanyl-L-valyl-L-arginyl-L-leucyl-L-phenylalanyl-L-isoleucyl-L-alpha-glutamyl-L-tryptophyl-L-leucyl-L-lysyl-L-asparagyl-glycyl-glycyl-L-prolyl-L-seryl-L-seryl-glycyl-L-alanyl-L-prolyl-L-prolyl-L-seryl-L-lysyl-L-lysyl-L-lysyl-L-lysyl-L-lysyl-L-lysinamide |
| SequenceShortening | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK; H-HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPSKKKKKK-[NH2] |
| Appearance | White to off-white solid powder |
| LogP | -30.8 |
| Hydrogen Bond Donor Count | 70 |
| Hydrogen Bond Acceptor Count | 77 |
| Rotatable Bond Count | 170 |
| Heavy Atom Count | 342 |
| Complexity | 11800 |
| Defined Atom Stereocenter Count | 42 |
| SMILES | S(C)CC[C@@H](C(N[C@@H](CCC(=O)O)C(N[C@@H](CCC(=O)O)C(N[C@@H](CCC(=O)O)C(N[C@@H](C)C(N[C@H](C(N[C@@H](CCCNC(=N)N)C(N[C@H](C(N[C@@H](CC1C=CC=CC=1)C(N[C@H](C(N[C@@H](CCC(=O)O)C(N[C@@H](CC1=CNC2C=CC=CC1=2)C(N[C@@H](CC(C)C)C(N[C@@H](CCCCN)C(N[C@@H](CC(N)=O)C(NCC(NCC(N1CCC[C@H]1C(N[C@@H](CO)C(N[C@@H](CO)C(NCC(N[C@@H](C)C(N1CCC[C@H]1C(N1CCC[C@H]1C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N[C@H](C(N)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CCCCN)=O)CO)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)[C@@H](C)CC)=O)=O)CC(C)C)=O)=O)C(C)C)=O)=O)=O)=O)=O)NC([C@H](CCC(N)=O)NC([C@H](CCCCN)NC([C@H](CO)NC([C@H](CC(C)C)NC([C@H](CC(=O)O)NC([C@H](CO)NC([C@H]([C@@H](C)O)NC([C@H](CC1C=CC=CC=1)NC([C@H]([C@@H](C)O)NC(CNC([C@H](CCC(=O)O)NC(CNC([C@H](CC1=CN=CN1)N)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O |
| InChi Key | XVVOERDUTLJJHN-IAEQDCLQSA-N |
| InChi Code | InChI=1S/C215H347N61O65S/c1-16-115(10)173(210(337)256-141(68-74-170(299)300)194(321)261-148(94-122-98-232-126-50-24-23-49-124(122)126)199(326)258-143(89-111(2)3)196(323)247-134(58-32-40-83-223)189(316)262-149(96-160(226)285)180(307)235-100-161(286)233-104-165(290)274-85-42-60-156(274)207(334)267-154(108-280)206(333)265-151(105-277)181(308)237-101-162(287)239-117(12)213(340)276-87-44-62-158(276)214(341)275-86-43-61-157(275)208(335)268-153(107-279)204(331)249-132(56-30-38-81-221)187(314)246-131(55-29-37-80-220)186(313)245-130(54-28-36-79-219)185(312)244-129(53-27-35-78-218)184(311)243-128(52-26-34-77-217)183(310)242-127(176(227)303)51-25-33-76-216)272-201(328)146(92-120-45-19-17-20-46-120)260-197(324)144(90-112(4)5)257-190(317)135(59-41-84-231-215(228)229)255-209(336)172(114(8)9)271-177(304)116(11)240-182(309)138(65-71-167(293)294)251-192(319)139(66-72-168(295)296)252-193(320)140(67-73-169(297)298)253-195(322)142(75-88-342-15)254-191(318)137(63-69-159(225)284)250-188(315)133(57-31-39-82-222)248-203(330)152(106-278)266-198(325)145(91-113(6)7)259-200(327)150(97-171(301)302)263-205(332)155(109-281)269-212(339)175(119(14)283)273-202(329)147(93-121-47-21-18-22-48-121)264-211(338)174(118(13)282)270-164(289)103-236-179(306)136(64-70-166(291)292)241-163(288)102-234-178(305)125(224)95-123-99-230-110-238-123/h17-24,45-50,98-99,110-119,125,127-158,172-175,232,277-283H,16,25-44,51-97,100-109,216-224H2,1-15H3,(H2,225,284)(H2,226,285)(H2,227,303)(H,230,238)(H,233,286)(H,234,305)(H,235,307)(H,236,306)(H,237,308)(H,239,287)(H,240,309)(H,241,288)(H,242,310)(H,243,311)(H,244,312)(H,245,313)(H,246,314)(H,247,323)(H,248,330)(H,249,331)(H,250,315)(H,251,319)(H,252,320)(H,253,322)(H,254,318)(H,255,336)(H,256,337)(H,257,317)(H,258,326)(H,259,327)(H,260,324)(H,261,321)(H,262,316)(H,263,332)(H,264,338)(H,265,333)(H,266,325)(H,267,334)(H,268,335)(H,269,339)(H,270,289)(H,271,304)(H,272,328)(H,273,329)(H,291,292)(H,293,294)(H,295,296)(H,297,298)(H,299,300)(H,301,302)(H4,228,229,231)/t115-,116-,117-,118+,119+,125-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,141-,142-,143-,144-,145-,146-,147-,148-,149-,150-,151-,152-,153-,154-,155-,156-,157-,158-,172-,173-,174-,175-/m0/s1 |
| Chemical Name | (4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-4-amino-1-[[2-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[(2S)-2-[(2S)-2-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1,6-diamino-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-2-oxoethyl]amino]-1,4-dioxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid |
| Synonyms | AVE0010Adlyxin; Lyxumia; ZP10A peptide; Lixisenatide Acetate; 1997361-87-1; Lixisenatide acetate (320367-13-3 free base); ZP10 A peptide; ZP10-A peptide; AVE-0010; AVE 0010 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GLP-1 receptor | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
Lixisenatide, shown in in vitro receptor binding studies, is a short-acting agonist of the glucagon-like peptide-1 receptor (GLP-1R), with an IC50 value of 1.4 nM for the human GLP-1 receptor. Receptor binding studies demonstrated that the affinity of Lixisenatide/ZP10A for the human GLP-1 receptor was 4-fold greater than the affinity of GLP-1 (7-36) amide.
GLP-1 Receptor Binding Studies. [2] In short, CHO-K1 cells harboring the human recombinant GLP-1 receptor were harvested. The membrane fraction containing the receptor was purified and used for... Binding of ZP10A to Human GLP-1 Receptor. The concentration resulting in half-maximal inhibition (IC50) of binding to the human GLP-1 receptor expressed in CHO-K1 cells was 5.5 ± 1.3 nM for GLP-1 (7-36) amide, a value within the range of those reported for GLP-1 binding to the endogenous receptor found in islet cell lines and to the recombinant receptor expressed in COS-7 cells (Goke and Conlon, 1988; Goke et al., 1989; Fehmann and Habener, 1991; Thorens, 1992; Wheeler et al., 1993). The IC50... |
|
| Cell Assay | Lixisenatide has the ability to prevent apoptosis caused by lipids and cytokines in Ins-1 cells, a β-cell line derived from rats. More significantly, lixisenatide can maintain insulin synthesis, storage, and pancreatic β-cell function in vitro and prevent lipotoxicity-induced insulin depletion in human islets. | |
| Animal Protocol |
Pphosphate-buffered saline, pH 7.4; 0.01, 0.1, 1, 10, and 100 nmol/kg; i.p. Male db/db mice C57BLKS/J-Leprdb/Leprdb ZP10A demonstrated dose-dependent improvement of glucose tolerance with an ED50 value of 0.02 nmol/kg i.p. in an oral glucose tolerance test (OGTT) in diabetic db/db mice. After 42 days of treatment, ZP10A dose-dependently (0, 1, 10, or 100 nmol/kg b.i.d.; n = 10/group), decreased glycosylated hemoglobin (HbA1C) from 8.4 +/- 0.4% (vehicle) to a minimum of 6.2 +/- 0.3% (100 nmol/kg b.i.d.; p < 0.05 versus vehicle) in db/db mice. Fasting blood glucose (FBG), glucose tolerance after an OGTT, and HbA1C levels were significantly improved in mice treated with ZP10A for 90 days compared with vehicle-treated controls. Interestingly, these effects were preserved 40 days after drug cessation in db/db mice treated with ZP10A only during the first 50 days of the study. Real-time polymerase chain reaction measurements demonstrated that the antidiabetic effect of early therapy with ZP10A was associated with an increased pancreatic insulin mRNA expression relative to vehicle-treated mice. In conclusion, long-term treatment of diabetic db/db mice with ZP10A resulted in a dose-dependent improvement of FBG, glucose tolerance, and blood glucose control. Our data suggest that ZP10A preserves beta-cell function. ZP10A is considered one of the most promising new drug candidates for preventive and therapeutic intervention in type 2 diabetes.[2] |
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following subcutaneous administration, the median Tmax of lixisenatide ranged from 1-3.5 hours, with no clinically relevant differences in the rate of absorption noted between possible injection sites (i.e. thigh, abdomen, or arm). Lixisenatide is presumably eliminated via glomerular filtration and proteolytic degradation. The apparent volume of distribution following subcutaneous administration is approximately 100 L. The mean apparent clearance of lixisenatide is approximately 35 L/h. Metabolism / Metabolites Lixisenatide is likely catabolized via non-specific proteolytic degradation. Biological Half-Life Following the administration of multiple doses in patients with type II diabetes mellitus, the mean terminal half-life of lixisenatide was approximately 3 hours. |
|
| Toxicity/Toxicokinetics |
Hepatotoxicity In large clinical trials, serum enzyme elevations were no more common with lixisenatide therapy than with placebo or comparator agents. In pooled safety analyses of more than 5000 patients, ALT elevations above 3 times the upper limit of normal occurred in 0.6% of both lixisenatide and placebo groups and no instances of treatment related clinically apparent liver injury were reported. Since licensure, there have been no published case reports of hepatotoxicity due to lixisenatide and the product label does not list liver injury as an adverse event. Thus, liver injury due to lixisenatide, as with other GLP-1 analogues, must be rare, if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of lixisenatide during breastfeeding. Because lixisenatide is a large peptide molecule with a molecular weight of 4858 daltons, the amount in milk is likely to be very low and absorption is unlikely because it is probably destroyed in the infant's gastrointestinal tract. Until more data become available, lixisenatide should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Lixisenatide is approximately 55% bound to human plasma proteins. |
|
| References |
[1]. Core Evid. 2011:6:67-79. [2]. J Pharmacol Exp Ther. 2003 Nov;307(2):490-6. [3]. Regul Pept. 2010 Sep 24;164(2-3):58-64. [4]. Diabetes Ther. 2016 Jun 18. [5]. Regul Pept. 2013 Aug 10;185:1-8. |
|
| Additional Infomation |
Lixisenatide is a forty-four membered polypeptide consisting of L-His, Gly, L-Glu, Gly, L-Thr, L-Phe, L-Thr, L-Ser, L-Asp, L-Leu, L-Ser, L-Lys, L-Gln, L-Met, L-Glu, L-Glu, L-Glu, L-Ala, L-Val, L-Arg, L-Leu, L-Phe, L-Ile, L-Glu, L-Trp, L-Leu, L-Lys, L-Asn, Gly, Gly, LPro, L-Ser, L-Ser, Gly, L-Ala, L-Pro, L-Pro, L-Ser, L-Lys, L-Lys, L-Lys, L-Lys, L-Lys, and L-Lys-NH2 residues joined in sequence. Used as an adjunct to diet and exercise for the treatment of adults with type II diabetes. It has a role as a glucagon-like peptide-1 receptor agonist, a hypoglycemic agent and a neuroprotective agent. It is a polypeptide and a peptidyl amide. Lixisenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist used in the treatment of type II diabetes mellitus (T2DM). It is sold by Sanofi-Aventis under the brand name Adlyxin in the US and Lyxumia in the EU. Adlyxin recieved FDA approval July 28, 2016. Lixisenatide is a recombinant DNA produced polypeptide analogue of human glucagon-like peptide-1 (GLP-1) which is used in combination with diet and exercise in the therapy of type 2 diabetes, either alone or in combination with other antidiabetic agents. Therapy with lixisenatide has not been associated with serum enzyme elevations or with episodes of clinically apparent liver injury. See also: Insulin Glargine; Lixisenatide (component of). Drug Indication Lixisenatide is indicated as an adjunct to diet and exercise to improve glycemic control in adult patients with type II diabetes mellitus. It is also available in combination with [insulin glargine] for the same indication. Lyxumia is indicated for the treatment of adults with type 2 diabetes mellitus to achieve glycaemic control in combination with oral glucose lowering medicinal products and/or basal insulin when these, together with diet and exercise, do not provide adequate glycaemic control. , Treatment of type II diabetes mellitus Mechanism of Action The activation of the GLP-1 receptor by lixisenatide results in the activation of adenylyl cyclase. This increases the concentration of cyclic adenosine monophosphate in the cell leading to the activation of protein kinase A (PKA) as well as Epac1 and Epac2. PKA, Epac1, and Epac2 are involved the in release of Ca2+ from the endoplasmic reticulum which is known as the "amplification" pathway which increases insulin release when the triggering pathway is activated. By activating this amplification pathway lixisenatide increases glucose stimulated insulin secretion. Lixisenatide is a once-daily glucagon-like peptide 1 (GLP-1) receptor agonist mimicking several favorable actions of endogenous GLP-1 that result in improved glycemic control with little or no hypoglycemia and weight loss. Phase II trials have shown that lixisenatide 20 μg once daily restores first-phase insulin release in patients with type 2 diabetes and improves the second-phase insulin response. Administered once or twice daily for 4 weeks, it significantly reduced postprandial and fasting blood glucose levels, and glycosylated hemoglobin (HbA(1c)). The efficacy and safety of lixisenatide once daily is being assessed in the GETGOAL Phase III clinical trial program. Results have shown beneficial effects on HbA(1c) compared with placebo in combination with commonly used antidiabetes agents, with no increased risk of hypoglycemia and with beneficial weight reduction. Adverse effects were similar to those observed for available GLP-1 receptor agonists, the most frequent being gastrointestinal. Both GLP-1 receptor agonists and long-acting insulin analogs have demonstrated protective effects on beta cells in preclinical studies. This, along with the pronounced effect of lixisenatide on postprandial plasma glucose, provides a rationale for combining it with long-acting basal insulin analogs, in the hope that the additive effects on glycemic control combined with a potential benefit on islet cells may lead to a new treatment approach to control blood glucose better and prevent long-term complications in patients with type 2 diabetes.[1] The glucagon-like peptide-1 (GLP-1) receptor represents an established therapeutic target in type 2 diabetes mellitus (T2DM). Agents that activate this receptor improve glucose tolerance alongside a low risk of hypoglycaemia, and have the potential to modify disease progression. Lixisenatide is a new potent and selective GLP-1 receptor agonist currently in development. The preclinical pharmacological profile of Lixisenatide suggests actions that are highly relevant to the long-term maintenance of glucose homeostasis. Lixisenatide protected Ins-1 cells (a rat-derived beta-cell line) from both lipid- and cytokine-induced apoptosis. More importantly, Lixisenatide also prevented lipotoxicity-induced insulin depletion in human islets and preserved insulin production, storage and pancreatic beta-cell function in vitro. Enhancement of insulin biosynthesis and pancreatic beta-cell volume could also be demonstrated in animal models of type 2 diabetes. The improvement of glucose-stimulated insulin secretion provided by Lixisenatide occurred in a strictly glucose-dependent manner. In animal models of diabetes, Lixisenatide improved basal blood glucose and HbA(1c) with a rapid onset and sustained duration of action, and prevented the deterioration of pancreatic responsiveness and glucose homeostasis. Lixisenatide also delayed gastric emptying and reduced food intake. The efficacy/safety profile of Lixisenatide is currently being studied further in an extensive ongoing Phase III clinical study programme. This article reviews the preclinical pharmacological profile of Lixisenatide.[3] Introduction: The extent to which postprandial glucagon reductions contribute to lowering of postprandial glucose in patients with type 2 diabetes mellitus (T2DM) is currently unknown. The aim of this analysis was to determine whether a reduction in postprandial glucagon following treatment with the glucagon-like peptide-1 receptor agonist lixisenatide correlates with a reduction in postprandial glucose and glycated hemoglobin (HbA1c) in patients with T2DM. Methods: A post hoc analysis was performed on pooled data from the modified intent-to-treat populations of two lixisenatide Phase 3 trials: GetGoal-M (lixisenatide versus placebo as add-on to metformin) and GetGoal-S (lixisenatide versus placebo as add-on to sulfonylurea [SU] ± metformin). Glucagon levels were assessed 2 h after a standardized meal test performed at baseline and Week 24 and were examined for correlation with changes in 2-h postprandial glucose and HbA1c. Results: Lixisenatide reduced 2-h postprandial glucagon at Week 24 compared with placebo (P < 0.00001). The mean change in postprandial glucagon significantly correlated with reductions in postprandial glucose (P < 0.00001) and HbA1c (P < 0.00001). Conclusion: A reduction in postprandial glucagon following lixisenatide administration correlated with a decrease in postprandial glucose and HbA1c in patients with T2DM insufficiently controlled on metformin and/or SU. This suggests that lowering of postprandial glucagon contributes to the overall glycemic improvement observed with lixisenatide.[4] Objectives: To determine the effects of lixisenatide, a new once-daily (QD) glucagon-like peptide-1 receptor agonist, on postprandial glucose (PPG) and gastric emptying, and the relationship between these effects in patients with type 2 diabetes mellitus (T2DM). Methods: Data were obtained from a randomized, double-blind, placebo-controlled, parallel-group study with treatment duration of 28 days in patients with T2DM receiving ≤2 oral antidiabetic drugs. Lixisenatide was injected subcutaneously using an ascending dose range (5-20 μg) increased every fifth day in increments of 2.5 μg. Blood glucose was determined before and after three standardized meals (breakfast, lunch, and dinner). Gastric emptying of the standardized breakfast was determined by a (13)C-octanoic acid breath test at baseline (Day-1) and at Day 28. Results: A total of 21 and 22 patients were randomized to lixisenatide 20 μg QD and placebo, respectively. With lixisenatide 20 μg QD, there was a reduction in PPG when compared with placebo after breakfast (p<0.0001), lunch (p<0.001) and dinner (p<0.05). Hence, lixisenatide 20 μg administered in the morning exhibited a pharmacodynamic effect on blood glucose throughout the day. Gastric emptying (50% emptying time) increased substantially from baseline with lixisenatide 20 μg QD, but not with placebo (change from baseline ± SD: -24.1 ± 133.1 min for placebo and 211.5 ± 278.5 min for lixisenatide; p<0.01). There was an inverse relationship between PPG area under the curve after breakfast and gastric emptying with lixisenatide 20 μg QD (n=17, r(2)=0.51, p<0.05), but not with placebo. Conclusions: In this study, lixisenatide at a dose of 20 μg QD reduced postprandial glycemic excursions in patients with T2DM, possibly as a result of sustained slowing of gastric emptying.[5] |
Solubility Data
| Solubility (In Vitro) | H2O: ~50 mg/mL (~9.6 mM) |
| Solubility (In Vivo) |
Note: Please refer to the "Guidelines for Dissolving Peptides" section in the 4th page of the "Instructions for use" file (upper-right section of this webpage) for how to dissolve peptides. Solubility in Formulation 1: 100 mg/mL (19.16 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.2058 mL | 1.0291 mL | 2.0583 mL | |
| 5 mM | 0.0412 mL | 0.2058 mL | 0.4117 mL | |
| 10 mM | 0.0206 mL | 0.1029 mL | 0.2058 mL |