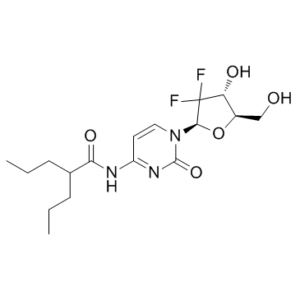
LY2334737 is the orally bioavailable prodrug of gemcitabine that is cleaved systemically to gemcitabine, a nucleoside anticancer drug used in chemotherapy. LY2334737 is an orally administered valproic acid ester of gemcitabine, a broad-spectrum antimetabolite with antineoplastic activity.After activation, oral administration of LY2334737 results in anticancer activity. By avoiding hydrolysis in enterocytes and the portal circulation, LY2334737 is able to increase systemic gemcitabine availability by avoiding first pass metabolism, in contrast to gemcitabine. Carboxylesterase 2 (CES2) hydrolyzes LY2334737, releasing gemcitabine systemically over a timescale that is consistent with formation rate-limited kinetics. Gemcitabine is subsequently transformed by deoxycytidine kinase into the active metabolites difluorodeoxycytidine triphosphate and diphosphate (dFdCDP and dFdCTP). Because dFdCDP inhibits ribonucleotide reductase, less deoxynucleotide is available for DNA replication. When dFdCTP is incorporated into DNA, DNA replication ends prematurely and eventually apoptosis is induced.
Physicochemical Properties
| Molecular Formula | C17H25F2N3O5 | |
| Molecular Weight | 389.39 | |
| Exact Mass | 389.176 | |
| Elemental Analysis | C, 52.44; H, 6.47; F, 9.76; N, 10.79; O, 20.54 | |
| CAS # | 892128-60-8 | |
| Related CAS # |
|
|
| PubChem CID | 11646777 | |
| Appearance | White to off-white solid powder | |
| LogP | 1.118 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 27 | |
| Complexity | 620 | |
| Defined Atom Stereocenter Count | 3 | |
| SMILES | FC1([C@H](O)[C@@H](CO)O[C@H]1N1C=CC(NC(=O)C(CCC)CCC)=NC1=O)F |
|
| InChi Key | MEOYFIHNRBNEPI-UXIGCNINSA-N | |
| InChi Code | InChI=1S/C17H25F2N3O5/c1-3-5-10(6-4-2)14(25)20-12-7-8-22(16(26)21-12)15-17(18,19)13(24)11(9-23)27-15/h7-8,10-11,13,15,23-24H,3-6,9H2,1-2H3,(H,20,21,25,26)/t11-,13-,15-/m1/s1 | |
| Chemical Name | N-[1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]-2-propylpentanamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Enterovirus A71 (EV-A71) |
| ln Vitro | LY2334737 treatment produced a response in five CES2-expressing cell lines. SK-OV-3 CES2 knockdown cells are less cytotoxic to LY2334737 than to parental cells. The level of CES2 expression was correlated with the drug response of HCT-116 cells transfected with CES2. Bystander investigations reveal that when cells are cocultured with CES2 rather than mock transfectants, LY2334737 statistically significantly inhibits PC-3-GFP growth[1]. |
| ln Vivo | Tumor growth inhibition of CES2 transfectant is higher than that of mock transfectant when 3.2 mg/kg LY2334737 is administered orally to xenograft models once daily for 21 days|1. In luciferase-tagged LM2-4 tumor xenografts, metronomic LY2334737 administration results in increased blood flow. This effect can be easily measured using contrast micro-ultrasound, and it coincides with a relative increase in tumor bioluminescence[3]. |
| References |
[1]. Human carboxylesterase-2 hydrolyzes the prodrug of gemcitabine (LY2334737) and confers prodrug sensitivity to cancer cells. Clin Cancer Res. 2013 Mar 1;19(5):1159-68. [2]. Drug Repurposing of Pyrimidine Analogs as Potent Antiviral Compounds Against Human Enterovirus A71 Infection With Potential Clinical Applications. Sci Rep. 2020 May 18;10(1):8159. [3]. Low-dose metronomic oral dosing of a prodrug of gemcitabine (LY2334737) causes antitumor effects in the absence of inhibition of systemic vasculogenesis. Mol Cancer Ther. 2012 Mar;11(3):680-9. |
| Additional Infomation |
LY2334737 has been used in trials studying the treatment of Solid Tumor, Metastatic Tumor, and Malignant Solid Tumor. Gemcitabine Prodrug LY2334737 is an orally available valproic acid prodrug of gemcitabine, a broad-spectrum antimetabolite and deoxycytidine analogue with antineoplastic activity. Upon administration, gemcitabine prodrug LY2334737 is hydrolyzed by carboxylesterase 2 (CES2) and releases gemcitabine systemically over a period of time consistent with formation rate-limited kinetics. In turn, gemcitabine is converted into the active metabolites difluorodeoxycytidine diphosphate and triphosphate (dFdCDP and dFdCTP) by deoxycytidine kinase. dFdCDP inhibits ribonucleotide reductase, thereby decreasing the deoxynucleotide pool available for DNA replication; dFdCTP is incorporated into DNA, resulting in premature termination of DNA replication and eventually the induction of apoptosis. Compared to gemcitabine, this prodrug is able to avoid hydrolysis in enterocytes and the portal circulation thus avoiding first pass metabolism and increasing systemic gemcitabine availability. In addition, the slow release of gemcitabine may enhance efficacy while lowering toxicity. CES2, a serine ester hydrolase, is expressed in certain tumors which may allow for increased conversion of gemcitabine at the tumor site thus increases cytotoxicity. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.42 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.42 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.42 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5681 mL | 12.8406 mL | 25.6812 mL | |
| 5 mM | 0.5136 mL | 2.5681 mL | 5.1362 mL | |
| 10 mM | 0.2568 mL | 1.2841 mL | 2.5681 mL |