Physicochemical Properties
| Molecular Formula | C9H15N3O5 |
| Molecular Weight | 245.23 |
| Exact Mass | 245.101 |
| Elemental Analysis | C, 44.08; H, 6.17; N, 17.13; O, 32.62 |
| CAS # | 948840-25-3 |
| Related CAS # | 1078151-47-9 (dihydrate); 948840-25-3 |
| PubChem CID | 135564845 |
| Appearance | White to off-white solid powder |
| Density | 1.64±0.1 g/cm3 (20 °C, 760 mmHg) |
| Boiling Point | 672.9±65.0 °C (760 mmHg) |
| Melting Point | 101 °C |
| LogP | -2.4 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 17 |
| Complexity | 275 |
| Defined Atom Stereocenter Count | 3 |
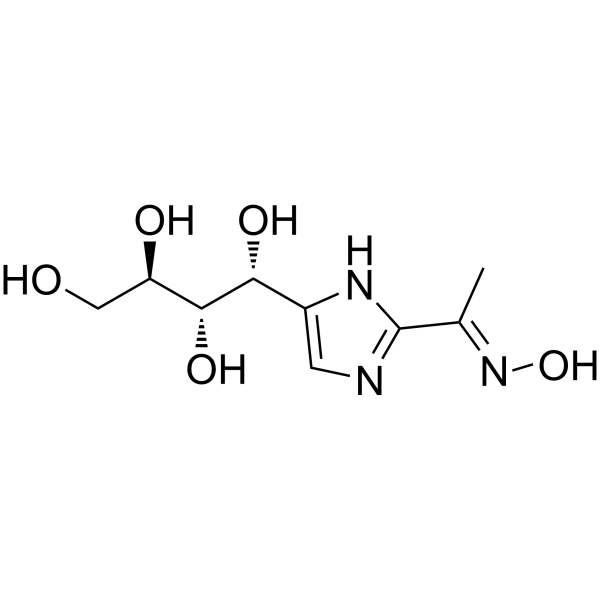
| SMILES | O[C@H]([C@@H](CO)O)[C@@H](C1=CN=C(/C(/C)=N/O)N1)O |
| InChi Key | AMXVYJYMZLDINS-RSWLNLDNSA-N |
| InChi Code | InChI=1S/C9H15N3O5/c1-4(12-17)9-10-2-5(11-9)7(15)8(16)6(14)3-13/h2,6-8,13-17H,3H2,1H3,(H,10,11)/b12-4+/t6-,7-,8-/m1/s1 |
| Chemical Name | (1R,2S,3R)-1-[2-[(E)-N-hydroxy-C-methylcarbonimidoyl]-1H-imidazol-5-yl]butane-1,2,3,4-tetrol |
| Synonyms | 948840-25-3; LX2931; LX-2931; LX3305; LX 2931; LX-3305; C5AGI979T7; CHEMBL1852164; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | S1PL/Sphingosine 1-Phosphate Lyase |
| ln Vivo |
Sphingosine 1-phosphate lyase (S1PL) has been characterized as a novel target for the treatment of autoimmune disorders using genetic and pharmacological methods. Medicinal chemistry efforts targeting S1PL by direct in vivo evaluation of synthetic analogues of 2-acetyl-4(5)-(1(R),2(S),3(R),4-tetrahydroxybutyl)-imidazole (THI, 1) led to the discovery of 2 (LX2931) and 4 (LX2932). The immunological phenotypes observed in S1PL deficient mice were recapitulated by oral administration of 2 or 4. Oral dosing of 2 or 4 yielded a dose-dependent decrease in circulating lymphocyte numbers in multiple species and showed a therapeutic effect in rodent models of rheumatoid arthritis (RA). Phase I clinical trials indicated that 2, the first clinically studied inhibitor of S1PL, produced a dose-dependent and reversible reduction of circulating lymphocytes and was well tolerated at dose levels of up to 180 mg daily. Phase II evaluation of 2 in patients with active rheumatoid arthritis is currently underway.[1] Therapeutic treatment with CYM5520 and LX2931 clearly increased long bone and vertebral bone mass to impressive 3-5 fold over vehicle in osteopenic ovariectomized mice. As expected, lymphopenia was a side effect of LX2931, whereas none occurred with CYM5520. Consistent with an osteoanabolic effect, CYM5520 increased osteoblast number, osteoid surface and alkaline phosphatase area 2-3 fold over vehicle. Plasma concentrations of the osteoanabolic marker procollagen I C-terminal propeptide were also elevated by CYM5520 and LX2931. LX2931 but not yet CYM5520 increased cortical thickness and mechanical strength without affecting mineral density. Conclusion and implications: Treatment with a pharmacological S1P2 agonist corrected ovariectomy-induced osteopenia in mice by inducing new bone formation thus constituting a novel osteoanabolic approach to osteoporosis.[2] |
| Animal Protocol | Ovariectomized 12 weeks old C57Bl6J mice were purchased from Charles River Laboratories. Mice were housed in SPF cages without any pathogens and with access to mouse chow and water ad libitum. Treatment was started 5 weeks after OVX. 1E)-1-(4-((1R,2S,3R)-1,2,3,4-Tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) was synthesized according to the known procedure and administered with the drinking water at 200 mg/kg/day for 6 weeks. CYM5520 was administered intraperitoneally at 10 mg/kg/day for 5 consecutive days per week for 6 weeks. The total number of mice used in the whole study was 21. Every effort was taken to minimize the number of animals used and their suffering.[2] |
| References |
[1]. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1 R, 2 S, 3 R)-1, 2, 3, 4-tetrahydroxybutyl)-1 H-imidazol-2-yl) ethanone oxime (LX2931) and (1 R, 2 S, 3 R)-1-(2-(isoxazol-3-yl)-1 H-imidazol-4-yl) butane-1, 2, 3, 4-tetraol (LX2932)[J]. Journal of medicinal chemistry, 2010, 53(24): 8650-8662. [2]. Agonist-induced activation of the S1P receptor 2 constitutes a novel osteoanabolic therapy for the treatment of osteoporosis in mice. Bone. 2019 Aug;125:1-7. |
| Additional Infomation |
Drug Indication Investigated for use/treatment in rheumatoid arthritis, inflammatory disorders (unspecified), and autoimmune diseases. Osteoporosis is a worldwide epidemic but pharmacological agents to stimulate new bone formation are scarce. We have shown that increasing tissue levels of sphingosine-1-phosphate (S1P) by blocking its degradation by the S1P lyase has pronounced osteoanabolic effect in mouse osteoporosis models by stimulating osteoblast differentiation through the S1P receptor 2 (S1P2). However, S1P lyase inhibitors have side effects complicating potential clinical use. Here, we tested whether direct S1P2 engagement by the S1P2 agonist CYM5520 exerted osteoanabolic potential in estrogen deficiency-induced osteopenia in mice. We compared its efficacy to LX2931, a novel S1P lyase inhibitor currently tested in rheumatoid arthritis.[2] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0778 mL | 20.3890 mL | 40.7780 mL | |
| 5 mM | 0.8156 mL | 4.0778 mL | 8.1556 mL | |
| 10 mM | 0.4078 mL | 2.0389 mL | 4.0778 mL |