kobe2602 is a novel, potent and selective small-molecule inhibitor of Ras–Raf interaction identified by SBDD (structure based drug design); it exhibits competitively inhibits the binding of H-Ras·GTP to c-Raf-1 RBD with a Ki value of 149 ± 55 μM. Kobe2602 is an analog of Kobe0065 that efficiently suppresses H-ras(G12V)-transformed NIH 3T3 cells' anchorage-dependent and independent growth and induces apoptosis. This is accompanied by the down-regulation of downstream molecules like MEK/ERK, Akt, and RalA as well as an upstream molecule called Son of Sevenless. Furthermore, oral administration of Kobe2602 results in antitumor activity on a xenograft of human colon carcinoma SW480 cells carrying the K-ras(G12V) gene. Kobe2602 might act as a building block for the creation of more potent and selective Ras inhibitors.
Physicochemical Properties
| Molecular Formula | C14H9N5O4F4S |
| Molecular Weight | 419.31096 |
| Exact Mass | 419.031 |
| Elemental Analysis | C, 40.10; H, 2.16; F, 18.12; N, 16.70; O, 15.26; S, 7.65 |
| CAS # | 454453-49-7 |
| PubChem CID | 3827738 |
| Appearance | Light yellow solid powder |
| Density | 1.7±0.1 g/cm3 |
| Boiling Point | 450.8±55.0 °C at 760 mmHg |
| Flash Point | 226.5±31.5 °C |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.683 |
| LogP | 5.12 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 28 |
| Complexity | 568 |
| Defined Atom Stereocenter Count | 0 |
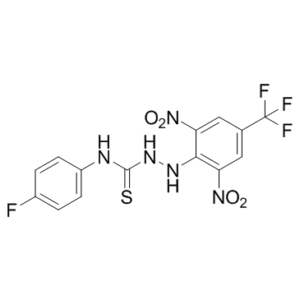
| SMILES | FC(C1=CC([N+]([O-])=O)=C(NNC(NC2=CC=C(F)C=C2)=S)C([N+]([O-])=O)=C1)(F)F |
| InChi Key | NNPBSITXCGPXJC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C14H9F4N5O4S/c15-8-1-3-9(4-2-8)19-13(28)21-20-12-10(22(24)25)5-7(14(16,17)18)6-11(12)23(26)27/h1-6,20H,(H2,19,21,28) |
| Chemical Name | 1-[2,6-dinitro-4-(trifluoromethyl)anilino]-3-(4-fluorophenyl)thiourea |
| Synonyms | Kobe 2602; Kobe2602; kobe2602; 454453-49-7; 2-[2,6-Dinitro-4-(trifluoromethyl)phenyl]-N-(4-fluorophenyl)hydrazinecarbothioamide; CHEMBL5280849; 1-[2,6-dinitro-4-(trifluoromethyl)anilino]-3-(4-fluorophenyl)thiourea; 3-{[2,6-dinitro-4-(trifluoromethyl)phenyl]amino}-1-(4-fluorophenyl)thiourea; 2-(2,6-dinitro-4-(trifluoromethyl)phenyl)-N-(4-fluorophenyl)hydrazinecarbothioamide; Kobe-2602 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | H-Ras·GTP; Ras-Raf interaction ( Ki = 149 μM ) |
| ln Vitro |
Kobe2602 (2–20 μM; 1 hour) shows Ras–Raf–binding inhibition in NIH 3T3 cells[1]. Kobe2602 inhibits cellular Ras-Raf binding with an IC50 value of roughly 10 μM[1]. Kobe2602 (20 μM) effectively suppresses the phosphorylation of Raf downstream kinases MEK and ERK in NIH 3T3 cells that are transiently expressing H-RasG12V[1]. Kobe2602 inhibits Ras⋅GTP but not Ras⋅GDP[1]. Kobe2602 (20 μM) prevents H-RasG12V-transformed cells from proliferating anchorage-dependently[1]. |
| ln Vivo | Kobe2602 (80 mg/kg; p.o.; five consecutive days per week; for 17 days) demonstrates antitumor activity against a xenograft of K-RasG12V-carrying human colon carcinoma SW480 cells[1]. |
| Enzyme Assay |
Biochemical Assays[1] H-Ras (residues 1–166) and GST-c-Raf-1-Rasbinding doman (RBD; residues 50–131) were produced in Escherichia coli and purified as described previously. For the in vitro binding inhibition assays, H-Ras(1–166), preloaded with [γ35S]GTPγS, was incubated with GST-c-Raf-1-RBD(50–131) at 25 °C for 30 min in the presence of the compound and the amount of bound H-Ras was quantified as the radioactivity pulled down by glutathione-sepharose resin. The Ki value for the compound was calculated as described in Fig. S1. For in vivo assays, NIH 3T3 cells were transfected with pEF-BOS-HA-H-RasG12V or pEF-BOS-HAK-RasG12V, cultured for 18 h at 10% (vol/vol) FBS, and then incubated in the presence of the compound at 2% (vol/vol) FBS for 1 h. Cells were lysed in 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, 1 mM DTT, phosphatase inhibitor mixture, and protease inhibitor mixture and subjected to detection of c-Raf-1 coimmunoprecipitated with an anti-H-Ras antibody (C-20) by Western blotting with an anti-cRaf-1 antibody (C-12), of phosphorylated MEK and ERK with anti-pMEK1/2 (p217/p221) and antiERK1/2 (p202/204) antibodies, and of phosphorylated Akt with an anti-pAkt antibody (Ser473) and of RalA·GTP pulled down with GST-Sec5(1–99) immobilized on glutathione-sepharose resin by an anti-RalA antibody. HA-tagged H-RasG12V·GTP was detected by an antiHA antibody. In vitro assays for the kinase activity of recombinant c-Raf-1 were performed by using a Raf-1 Kinase Assay kit. In vitro GDP–GTP exchange assays were done by incubating 600 nM GST-H-Ras(1–166)·GDP immobilized on glutathione-sepharose resin with 11 μM [γ35S]GTPγS (1,500 cpm/pmol) at 25 °C in the presence of purified 6×His-tagged mouse Son of sevenless (mSos)1(563–1,049) (180 nM each), wildtype, or a W729E mutant (5) in buffer B [50 mM Tris·HCl (pH 7.4), 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 20 mM imidazol]. The radioactivity remaining on the resin after an intensive washing was quantified by liquid scintillation counting. Varying concentrations of compounds were added to the reaction mixtures to observe their inhibitory effect. |
| Cell Assay |
Cell Line: H-rasG12V-transformed NIH 3T3 cells Concentration: 20 μM Incubation Time: 24 hours , 48 hours, 72 hours Result: Efficiently inhibited colony formation in soft agar in a dose-dependent manner. Colony Formation Assays.[1] Cells (103 to 104 ) were inoculated in 2 mL of DMEM containing 10% (vol/vol) FBS, 0.33% SeaPlaque agarose, and one of the compounds and overlaid onto bottom agar consisting of 4 mL of DMEM containing 10% (vol/vol) FBS, 0.6% SeaPlaque agarose, and the same concentration of the compound in a six-well culture plate. After incubation at 37 °C for 14–21 d, the number of colonies >200 μm in diameter was counted under a dissecting microscope. Cell Proliferation Assays.[1] Cells (2 × 103 ) were seeded in a 96-well plate and cultured in DMEM containing 2% (vol/vol) FBS in the presence of one of the compounds. Viable cell numbers were measured by formazan formation using a Cell Counting Kit 8. Apoptotic cells were detected by a standard TUNEL assay using an In Situ Cell Detection kit. |
| Animal Protocol |
Female athymic nude mice (6-8 wk old), with SW480 cells xenograft 80 mg/kg Oral administration, five consecutive days per week, for 17 days Tumor Xenografts. [1] Cells (5 × 106 ) were implanted into the right flanks of female athymic nude mice (6–8 wk old). After tumor sizes reached ∼50 mm3 on average, compounds (e.g. Kobe0065) suspended in Cremophor:ethanol:water (1:1:6) were administered orally for five consecutive days per week for 17 d. Tumor volumes (V) were calculated with the following formula: V = A × B2 /2, where A is the largest diameter and B is the perpendicular diameter. Dissected tumors after 17-d administration of the 80 mg/ kg compounds were fixed in 4% (wt/vol) paraformaldehyde and embedded in paraffin. Their sections were subjected to immunohistochemistry with an anti-ERK1/2 antibody or an anti-CD31 antibody using a HISTMOUSE-PLUS kit. Apoptotic cells were detected by a TUNEL assay. Statistical significance for groups of three or more was determined by one-way ANOVA with Tukey’s test for post hoc analysis. |
| References |
[1]. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proceedings of the National Academy of Sciences of the United States of America (2013), 110(20), 8182-8187, |
| Additional Infomation | Mutational activation of the Ras oncogene products (H-Ras, K-Ras, and N-Ras) is frequently observed in human cancers, making them promising anticancer drug targets. Nonetheless, no effective strategy has been available for the development of Ras inhibitors, partly owing to the absence of well-defined surface pockets suitable for drug binding. Only recently, such pockets have been found in the crystal structures of a unique conformation of Ras⋅GTP. Here we report the successful development of small-molecule Ras inhibitors by an in silico screen targeting a pocket found in the crystal structure of M-Ras⋅GTP carrying an H-Ras-type substitution P40D. The selected compound Kobe0065 and its analog Kobe2602 exhibit inhibitory activity toward H-Ras⋅GTP-c-Raf-1 binding both in vivo and in vitro. They effectively inhibit both anchorage-dependent and -independent growth and induce apoptosis of H-ras(G12V)-transformed NIH 3T3 cells, which is accompanied by down-regulation of downstream molecules such as MEK/ERK, Akt, and RalA as well as an upstream molecule, Son of sevenless. Moreover, they exhibit antitumor activity on a xenograft of human colon carcinoma SW480 cells carrying the K-ras(G12V) gene by oral administration. The NMR structure of a complex of the compound with H-Ras⋅GTP(T35S), exclusively adopting the unique conformation, confirms its insertion into one of the surface pockets and provides a molecular basis for binding inhibition toward multiple Ras⋅GTP-interacting molecules. This study proves the effectiveness of our strategy for structure-based drug design to target Ras⋅GTP, and the resulting Kobe0065-family compounds may serve as a scaffold for the development of Ras inhibitors with higher potency and specificity.[1] |
Solubility Data
| Solubility (In Vitro) |
DMSO: 14.3~84 mg/mL (34.1~200.3 mM) Ethanol: ~14 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (11.92 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (11.92 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3849 mL | 11.9244 mL | 23.8487 mL | |
| 5 mM | 0.4770 mL | 2.3849 mL | 4.7697 mL | |
| 10 mM | 0.2385 mL | 1.1924 mL | 2.3849 mL |