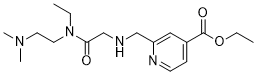
KDM5-C70 is a novel and potent JARID1 (Jumonji AT-Rich Interactive Domain 1) Histone Demethylases Inhibitor with anticancer activity. It is an ethyl ester prodrug of KDM5-C49 with enhanced cellular permeability.
Physicochemical Properties
| Molecular Formula | C17H28N4O3 |
| Molecular Weight | 336.436 |
| Exact Mass | 336.216 |
| Elemental Analysis | C, 60.69; H, 8.39; N, 16.65; O, 14.27 |
| CAS # | 1596348-32-1 |
| PubChem CID | 90094283 |
| Appearance | Light yellow to yellow liquids |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 466.6±45.0 °C at 760 mmHg |
| Flash Point | 236.0±28.7 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.525 |
| LogP | 0.75 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 24 |
| Complexity | 390 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(C([H])([H])N([H])C([H])([H])C1C([H])=C(C(=O)OC([H])([H])C([H])([H])[H])C([H])=C([H])N=1)N(C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H] |
| InChi Key | WCILOMUUNVPIKQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C17H28N4O3/c1-5-21(10-9-20(3)4)16(22)13-18-12-15-11-14(7-8-19-15)17(23)24-6-2/h7-8,11,18H,5-6,9-10,12-13H2,1-4H3 |
| Chemical Name | ethyl 2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-oxoethyl)amino)methyl)isonicotinate |
| Synonyms | KDM5 C70 KDM5C70 KDM5-C70 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | KDM5 histone demethylase |
| ln Vitro | After 7 days of treatment at increasing concentrations, KDM5-C70 (10-9-10-5 M; 7 days; MM.1S myeloma cells) therapy demonstrated antiproliferative effects (expectedly, KDM5-C70 lowered viability/proliferation at ~20 50%) micron)[1]. Treatment with KDM5-C70 (50 μM; 7 days; MM.1S myeloma cells) reduced retinoblastoma protein (Rb) phosphorylation levels, but did not affect overall levels of phosphorylated Rb (pRb), suggesting poor cell cycle progress [1]. At a 50 μM inhibitor dose, chromatin immunoprecipitation and next-generation sequencing revealed an increase in H3K4me3 levels surrounding the KDM5-C70 transcription start site, although GSK467A did not alter appreciably [1]. |
| Enzyme Assay | Members of the KDM5 (also known as JARID1) family are 2-oxoglutarate- and Fe(2+)-dependent oxygenases that act as histone H3K4 demethylases, thereby regulating cell proliferation and stem cell self-renewal and differentiation. Here we report crystal structures of the catalytic core of the human KDM5B enzyme in complex with three inhibitor chemotypes. These scaffolds exploit several aspects of the KDM5 active site, and their selectivity profiles reflect their hybrid features with respect to the KDM4 and KDM6 families. Whereas GSK-J1, a previously identified KDM6 inhibitor, showed about sevenfold less inhibitory activity toward KDM5B than toward KDM6 proteins, KDM5-C49 displayed 25-100-fold selectivity between KDM5B and KDM6B. The cell-permeable derivative KDM5-C70 had an antiproliferative effect in myeloma cells, leading to genome-wide elevation of H3K4me3 levels. The selective inhibitor GSK467 exploited unique binding modes, but it lacked cellular potency in the myeloma system. Taken together, these structural leads deliver multiple starting points for further rational and selective inhibitor design[1]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: MM.1S Myeloma Cells Tested Concentrations: 10-9-10-5 M Incubation Duration: 7 days Experimental Results: Antiproliferative effect was shown after 7 days of treatment at increasing concentrations. Western Blot Analysis[1] Cell Types: MM.1S Myeloma Cells Tested Concentrations: 50 μM Incubation Duration: 7 days Experimental Results: diminished phosphorylation levels of retinoblastoma protein (Rb). |
| References |
[1]. Structural analysis of human KDM5B guides histone demethylase inhibitor development. Nat Chem Biol. 2016 Jul;12(7):539-45. [2]. KDM5 lysine demethylases are involved in maintenance of 3'UTR length. Sci Adv. 2016 Nov 18;2(11):e1501662. |
| Additional Infomation | The complexity by which cells regulate gene and protein expression is multifaceted and intricate. Regulation of 3' untranslated region (UTR) processing of mRNA has been shown to play a critical role in development and disease. However, the process by which cells select alternative mRNA forms is not well understood. We discovered that the Saccharomyces cerevisiae lysine demethylase, Jhd2 (also known as KDM5), recruits 3'UTR processing machinery and promotes alteration of 3'UTR length for some genes in a demethylase-dependent manner. Interaction of Jhd2 with both chromatin and RNA suggests that Jhd2 affects selection of polyadenylation sites through a transcription-coupled mechanism. Furthermore, its mammalian homolog KDM5B (also known as JARID1B or PLU1), but not KDM5A (also known as JARID1A or RBP2), promotes shortening of CCND1 transcript in breast cancer cells. Consistent with these results, KDM5B expression correlates with shortened CCND1 in human breast tumor tissues. In contrast, both KDM5A and KDM5B are involved in the lengthening of DICER1. Our findings suggest both a novel role for this family of demethylases and a novel targetable mechanism for 3'UTR processing.[2] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~297.24 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.43 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.43 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.43 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9723 mL | 14.8615 mL | 29.7230 mL | |
| 5 mM | 0.5945 mL | 2.9723 mL | 5.9446 mL | |
| 10 mM | 0.2972 mL | 1.4861 mL | 2.9723 mL |