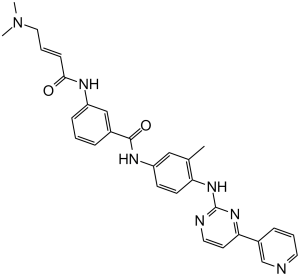
JNK-IN-8 (JNK Inhibitor XVI; JNK-IN8) is the first pan-JNK (c-Jun N-terminal Kinase) inhibitor that is irreversible and covalent and has potential antitumor activity. By covalently attaching to Cys116 in their catalytic sites and inhibiting JNK1/2/4 with IC50 values of 4.7 nM, 18.7 nM, and 1 nM. For investigating JNK-dependent signal transduction, JNK-IN-8 is a helpful probe.
Physicochemical Properties
| Molecular Formula | C29H29N7O2 |
| Molecular Weight | 507.59 |
| Exact Mass | 507.238 |
| Elemental Analysis | C, 68.62; H, 5.76; N, 19.32; O, 6.30 |
| CAS # | 1410880-22-6 |
| Related CAS # | JNK-IN-8;1410880-22-6 |
| PubChem CID | 57340686 |
| Appearance | White to yellow solid powder |
| Density | 1.3±0.1 g/cm3 |
| Index of Refraction | 1.686 |
| LogP | 2.79 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 38 |
| Complexity | 791 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C(C1C([H])=C([H])C([H])=C(C=1[H])N([H])C(/C(/[H])=C(\[H])/C([H])([H])N(C([H])([H])[H])C([H])([H])[H])=O)N([H])C1C([H])=C([H])C(=C(C([H])([H])[H])C=1[H])N([H])C1=NC([H])=C([H])C(C2=C([H])N=C([H])C([H])=C2[H])=N1 |
| InChi Key | GJFCSAPFHAXMSF-UXBLZVDNSA-N |
| InChi Code | InChI=1S/C29H29N7O2/c1-20-17-24(11-12-25(20)34-29-31-15-13-26(35-29)22-8-5-14-30-19-22)33-28(38)21-7-4-9-23(18-21)32-27(37)10-6-16-36(2)3/h4-15,17-19H,16H2,1-3H3,(H,32,37)(H,33,38)(H,31,34,35)/b10-6+ |
| Chemical Name | 3-[[(E)-4-(dimethylamino)but-2-enoyl]amino]-N-[3-methyl-4-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide |
| Synonyms | JNK Inhibitor XVI; JNK-IN 8; JNK-IN 8; 1410880-22-6; JNK Inhibitor XVI; JNK-IN-8 (GMP); 3-[[(E)-4-(dimethylamino)but-2-enoyl]amino]-N-[3-methyl-4-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide; (E)-3-(4-(dimethylamino)but-2-enamido)-N-(3-methyl-4-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide; Benzamide, 3-[[4-(dimethylamino)-1-oxo-2-buten-1-yl]amino]-N-[3-methyl-4-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-; 1644498-56-5; JNK-IN8; c-Jun N-terminal Kinase Inhibitor XVI |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets |
JNK3 (IC50 = 1 nM); JNK1 (IC50 = 4.7 nM); JNK2 (IC50 = 18.7 nM); Kit (V559D,T670I) (IC50 = 56 nM) JNK-IN-8 targets JNK1 (IC₅₀ = 4.7 nM), JNK2 (IC₅₀ = 18.7 nM), and JNK3 (IC₅₀ = 1 nM) through covalent binding to conserved cysteine residues [1] |
| ln Vitro |
JNK-IN-8 has an EC50 of 486 nM and 338 nM, respectively, in HeLa and A375 cells where it prevents c-Jun phosphorylation. IRAK1, PIK3C3, PIP4K2C, and PIP5K3 no longer bind to JNK-IN-8, which results in a notable improvement in selectivity. For JNK2 inhibition, Cys116 is necessary in JNK-IN-8.[1] JNK-IN-8 (10 mM) inhibits the JNKs' well-known substrate, IL-1R cells' induced phosphorylation of c-Jun. The covalent attachment of JNK-IN-8 to the JNK isoforms resulted in a slight reduction in the mobility of the JNK isoforms during electrophoresis.[2] JNK-IN-8 was found to inhibit JNK kinase by using the KinomeScan method to profile a library of acrylamide kinase inhibitors based on the structure of imatinib. JNK-IN-8 uses an N,N-dimethyl butenoic acetamide warhead to covalently target Cys154 and differs from imatinib in its regiochemistry of the 1,4-dianiline and 1,3-aminobenzoic acid substructures. To reach Cys 154, which is close to the lip of the ATP-binding site, JNK-IN-8 adopts an L-shaped type I binding conformation. [3] Kinase activity inhibition: JNK-IN-8 irreversibly inhibits JNK1/2/3 kinase activity by covalently binding to cysteine residues (e.g., Cys154 in JNK1), with IC₅₀ values of 4.7 nM, 18.7 nM, and 1 nM for JNK1, JNK2, and JNK3, respectively. It dose-dependently blocks phosphorylation of the downstream substrate c-Jun [1] Signal pathway regulation: In HEK-293 cells, JNK-IN-8 (0.1-3 μM) treatment for 3 hours reduces phosphorylation of JNK (T183/Y185) and c-Jun (Ser63/73), confirming inhibition of the JNK-c-Jun pathway [1] Anti-inflammatory activity: In LPS-stimulated primary mouse peritoneal macrophages, JNK-IN-8 (10 μM) pretreatment for 1 hour decreases mRNA expression and secretion of TNF-α, IL-6, and IL-1β. It also reduces malondialdehyde (MDA) levels and restores superoxide dismutase (SOD) activity, indicating anti-inflammatory and antioxidant effects [1] |
| ln Vivo | JNK-IN-8 is a novel and potent JNK inhibitor that specially targets JNK activation. It has anti-fungal activity. |
| Enzyme Assay |
Recombinant JNK1/2/3 proteins are incubated with ATP and substrates (e.g., c-Jun fragments) in reaction buffer, followed by addition of JNK-IN-8 (0.1-1000 nM). Substrate phosphorylation is measured via radiolabeling or fluorescence to assess inhibitory activity. Results show JNK-IN-8 exhibits the highest potency against JNK3 [1]. Kinetics of binding assay Pretreatment of A375 cells with 1 μM JNK-IN-8 is carried out for the specified times. Remove the medium and give it three PBS washes. Re-suspend the cell pellet in 1 mL of lysis buffer, which contains phosphatase inhibitor cocktail, protease inhibitor cocktail, 1% NP-40, 1% CHAPS, 25 mM Tris, and 150 mM NaCl. Rotate in a circle for 30 minutes at 4 °C. Lysates are removed by centrifugation in the Eppendorf at 1.4×104 rpm for 15 min. The cleared lysates are gel filtered through 10DG columns into Kinase Buffer (0.1% NP-40, 20 mM HEPES, 150 mM NaCl, Phosphatase Inhibitor Cocktail, and Protease Inhibitor Cocktail). The gel-filtered lysate's total protein concentration should be between 5 and 15 mg/ml. The probe from ActivX is used to label cell lysate for 1 hour at a 5 μM concentration. In order to get rid of extra reagents and change the buffer, samples are reduced with DTT, and cysteines are blocked with iodoacetamide. Add 50 μL of streptavidin bead slurry and 1 vol of 2× Binding Buffer (2% Triton-100, 1% NP-40, 2 mM EDTA, 2× PBS). Rotate end to end for 2 hours, and then centrifuge at 7,000 rpm for 2 minutes. Wash three times with PBS and three times with 1× Binding Buffer. Beads are given 30 μL 1× of sample buffer before being heated at 95°C for 10 minutes. Run samples at 110 volts through an SDS-PAGE gel. |
| Cell Assay |
Antiproliferative and apoptotic effects: In A375 melanoma cells, JNK-IN-8 (1-100 μM) treatment for 72 hours reduces cell viability in a dose-dependent manner (MTT assay). Annexin V-FITC/PI staining reveals increased early apoptotic cells and elevated caspase-3/7 activity after 48-hour treatment with 5-20 μM JNK-IN-8 [1] Stem cell expansion: In human umbilical cord blood CD34⁺ hematopoietic stem cells, 7-day treatment with 2 μM JNK-IN-8 increases the proportion of CD34⁺CD38⁻ stem cells (flow cytometry) and preserves long-term engraftment capacity [1]. Interleukin Receptor 1 (IL1R)-expressing HEK-293 cells are grown in DMEM, which has been supplemented with 10% FBS, 2 mM glutamine, and 1 antimycotic/antibiotic solution. Before being treated with DMSO or JNK-IN-8 or being stimulated with 2 M anisomycin for 1 hour, cells are serum starved for 18 hours[1]. Lysates are then clarified by centrifugation for 10 minutes at 16000 g and 4°C. |
| References |
[1]. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012 Jan 27;19(1):140-54. [2]. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol. 2013 Feb 21;20(2):146-59. |
| Additional Infomation |
3-[[4-(dimethylamino)-1-oxobut-2-enyl]amino]-N-[3-methyl-4-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]benzamide is a member of benzamides. JNK-IN-8 is the first reported covalent JNK inhibitor, acting via irreversible binding to JNK cysteine residues to block downstream signaling [1]. It shows potential for treating cancers (e.g., melanoma), inflammatory diseases, and enhancing hematopoietic stem cell expansion [1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.93 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (4.93 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.93 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly.. Solubility in Formulation 4: 2% DMSO+30% PEG 300+5% Tween 80+ddH2O: 10mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9701 mL | 9.8505 mL | 19.7009 mL | |
| 5 mM | 0.3940 mL | 1.9701 mL | 3.9402 mL | |
| 10 mM | 0.1970 mL | 0.9850 mL | 1.9701 mL |