JNJ-42153605 is a potent, selective and allosteric modulator of the mGlu2 (metabotropic glutamate 2) receptor with with an EC50 of 17 nM. It exhibits a superior pharmacokinetic profile in both rodent and nonrodent variants. It is determined that JNJ-42153605 is not an agonist or antagonist toward other mGlu receptor subtypes up to 30 μM when its selectivity for the mGlu2 receptor is evaluated. With no signs of P-glycoprotein efflux, JNJ-42153605 exhibits high permeability. Using a dose of 3 mg/kg po in the rat sleep-wake EEG paradigm, JNJ-42153605 demonstrated a central in vivo efficacy by inhibiting the REM sleep state, a phenomenon previously demonstrated to be mGlu2 mediated. Using an ED₩₀ of 5.4 mg/kg sc, which is suggestive of antipsychotic activity, JNJ-42153605 reversed PCP-induced hyperlocomotionin mice.
Physicochemical Properties
| Molecular Formula | C₂₂H₂₃F₃N₄ | |
| Molecular Weight | 400.44 | |
| Exact Mass | 400.187 | |
| Elemental Analysis | C, 65.99; H, 5.79; F, 14.23; N, 13.99 | |
| CAS # | 1254977-87-1 | |
| Related CAS # |
|
|
| PubChem CID | 49765871 | |
| Appearance | White to off-white solid powder | |
| LogP | 5.149 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 29 | |
| Complexity | 553 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | FC(C1=C(N2CCC(C3=CC=CC=C3)CC2)C=CN4C1=NN=C4CC5CC5)(F)F |
|
| InChi Key | BQAVZGJJQFJSMW-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C22H23F3N4/c23-22(24,25)20-18(10-13-29-19(14-15-6-7-15)26-27-21(20)29)28-11-8-17(9-12-28)16-4-2-1-3-5-16/h1-5,10,13,15,17H,6-9,11-12,14H2 | |
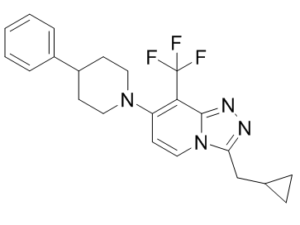
| Chemical Name | 3-(cyclopropylmethyl)-7-(4-phenylpiperidin-1-yl)-8-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyridine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mGluR2 ( EC50 = 17 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
|
|
| References |
[1]. Discovery of 3-cyclopropylmethyl-7-(4-phenylpiperidin-1-yl)-8-trifluoromethyl[1,2,4]triazolo[4,3-a]pyridine (JNJ-42153605): a positive allosteric modulator of the metabotropic glutamate 2 receptor. J Med Chem. 2012 Oct 25;55(20):8770-89. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 1.67 mg/mL (4.17 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.67 mg/mL (4.17 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1.67 mg/mL (4.17 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4973 mL | 12.4863 mL | 24.9725 mL | |
| 5 mM | 0.4995 mL | 2.4973 mL | 4.9945 mL | |
| 10 mM | 0.2497 mL | 1.2486 mL | 2.4973 mL |