JNJ16259685 (JNJ-16259685) is a novel, potent, selective and non-competitive antagonist of mGlu1 receptor with IC50 of 19 nM. JNJ16259685 did not alter immobility, but it did result in a notable decrease in offensive behaviors (attack and threat). In a concentration-dependent way, it prevents mGlu1 from being synaptically activated. Up to a dose of 30 mg/kg, JNJ16259685 had very little effect on hip flexion and posture. In rats, motor skill remained intact for well-learned tasks (up to 30 mg/kg), but in mice, it was impaired. The learning of a novel motor skill (rotarod) was severely hindered in both rats and mice rats (0.3 mg/kg).
Physicochemical Properties
| Molecular Formula | C20H23NO3 | |
| Molecular Weight | 325.41 | |
| Exact Mass | 325.168 | |
| Elemental Analysis | C, 73.82; H, 7.12; N, 4.30; O, 14.75 | |
| CAS # | 409345-29-5 | |
| Related CAS # |
|
|
| PubChem CID | 11313361 | |
| Appearance | White to off-white solid powder | |
| Density | 1.21g/cm3 | |
| Boiling Point | 502.5ºC at 760 mmHg | |
| Flash Point | 257.7ºC | |
| Index of Refraction | 1.604 | |
| LogP | 3.947 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 24 | |
| Complexity | 446 | |
| Defined Atom Stereocenter Count | 0 | |
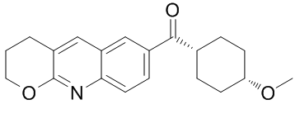
| SMILES | O=C(C1=CC=C2N=C3C(CCCO3)=CC2=C1)[C@H]4CC[C@@H](OC)CC4 |
|
| InChi Key | QOTAQTRFJWLFCR-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3 | |
| Chemical Name | 3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl-(4-methoxycyclohexyl)methanone | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mGluR1 ( IC50 = 19 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
Mice: There are nine mouse groups in use. The animals are randomized into seven experimental groups (N=14–16 each) that receive injections of JNJ16259685 and two control groups (n=15 each) that receive only saline or saline (90%) plus DMSO (10%). In order to provide suitable injection doses, JNJ16259685 is diluted in saline (90%) plus DMSO (10%) and given in seven doses: 0.125, 0.25, 0.5, 1, 2, 4, and 8 mg/kg. The dosages are selected based on the results of recent behavioral research with this substance. Ten milliliters per kilogram of drug or vehicle are injected intraperitoneally. Rats: The overt behavioral, neurological, and autonomic reactions to the drug challenge are measured using this procedure. In summary, rats are randomly assigned to four groups (n = 6), each of which is given a different dose of JNJ16259685 (0, 3, 10, or 30 mg/kg). The animals are evaluated and scored at 30, 60, 120, and 240 minutes after injection by a skilled observer who is blind to the drugs the animals are receiving. The animals are evaluated for gait, pupil size, body elevation, limb position, limb tone, and passivity. For every behavior, animals that seemed "normal" were given a score of 0, while animals that showed mild, moderate, or severe increases (+) or decreases (−) from normalcy were given scores of ±1, ±2, or ±3. Individual animals are deemed to be significantly affected on the measure if they score ±2 or higher. If three or more of the animals receive a score greater than ±2, the dose is deemed significant. |
|
| References |
[1]. JNJ16259685, a selective mGlu1 antagonist, suppresses isolation-induced aggression in male mice. Eur J Pharmacol. 2008 May 31;586(1-3):217-20. [2]. Characterization of the selective mGluR1 antagonist, JNJ16259685, in rodent models of movement and coordination. Pharmacol Biochem Behav. 2011 Apr;98(2):181-7. [3]. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004 Dec;47(7):961-72. [4]. Potent and Specific Action of the mGlu1 Antagonists YM-298198 and JNJ16259685 on Synaptic Transmission in Rat Cerebellar Slices. Br J Pharmacol. 2007 Jul;151(6):870-6. |
|
| Additional Infomation | 3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl-(4-methoxycyclohexyl)methanone is an organonitrogen heterocyclic compound, an oxacycle and an organic heterotricyclic compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.75 mg/mL (8.45 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.75 mg/mL (8.45 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.75 mg/mL (8.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 27.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0730 mL | 15.3652 mL | 30.7305 mL | |
| 5 mM | 0.6146 mL | 3.0730 mL | 6.1461 mL | |
| 10 mM | 0.3073 mL | 1.5365 mL | 3.0730 mL |