Physicochemical Properties
| Molecular Formula | C6H8O7 |
| Molecular Weight | 192.12 |
| Exact Mass | 192.027 |
| CAS # | 320-77-4 |
| Related CAS # | DL-Isocitric acid trisodium salt;1637-73-6 |
| PubChem CID | 1198 |
| Appearance | Typically exists as White to off-white solid at room temperature |
| Density | 1.751g/cm3 |
| Boiling Point | 329.6ºC at 760 mmHg |
| Melting Point | 162 - 165 °C |
| Flash Point | 167.4ºC |
| Index of Refraction | 1.569 |
| LogP | -1.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 13 |
| Complexity | 233 |
| Defined Atom Stereocenter Count | 0 |
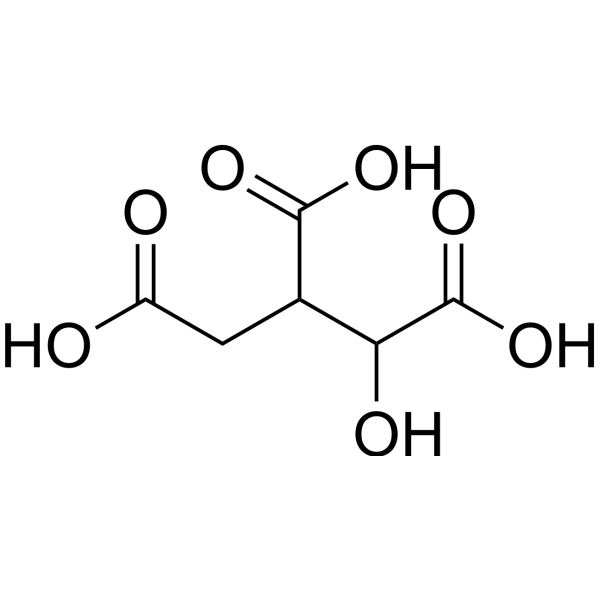
| SMILES | OC(CC(C(C(=O)O)O)C(=O)O)=O |
| InChi Key | ODBLHEXUDAPZAU-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C6H8O7/c7-3(8)1-2(5(10)11)4(9)6(12)13/h2,4,9H,1H2,(H,7,8)(H,10,11)(H,12,13) |
| Chemical Name | 1-hydroxypropane-1,2,3-tricarboxylic acid |
| Synonyms | isocitric acid; 320-77-4; isocitrate; 1-Hydroxypropane-1,2,3-tricarboxylic acid; 3-Carboxy-2,3-dideoxy-1-hydroxypropan-1,2,3-tricarboxylic acid; DL-Isocitric acid; 3-carboxy-2,3-dideoxypentaric acid; 1-Hydroxytricarballylic acid; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | Endogenous metabolites are those that the Kyoto Encyclopedia of Genes and Genomes has identified as products or substrates of the approximately 1900 metabolic enzymes that are encoded in human genome. Numerous of these metabolites have been shown to have harmful effects, as evidenced by the body of literature [1]. |
| References |
[1]. Endogenous toxic metabolites and implications in cancer therapy. Oncogene. 2020 Aug;39(35):5709-5720. [2]. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis. 2013 Oct;34(19):2865-72. [3]. Intracellular flux analysis applied to the effect of dissolved oxygen on hybridomas. Appl Microbiol Biotechnol. 1995 Dec;44(1-2):27-36. |
| Additional Infomation |
Isocitric acid is a tricarboxylic acid that is propan-1-ol with a hydrogen at each of the 3 carbon positions replaced by a carboxy group. It has a role as a fundamental metabolite. It is a tricarboxylic acid and a secondary alcohol. It is a conjugate acid of an isocitrate(1-). Isocitric acid is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Isocitric acid has been reported in Populus tremula, Vaccinium macrocarpon, and other organisms with data available. Isocitric acid is a metabolite found in or produced by Saccharomyces cerevisiae. It is well recognized that many metabolic enzymes play essential roles in cancer cells in producing building blocks such as nucleotides, which are required in greater amounts due to their increased proliferation. On the other hand, the significance of enzymes in preventing the accumulation of their substrates is less recognized. Here, we outline the evidence and underlying mechanisms for how many metabolites normally produced in cells are highly toxic, such as metabolites containing reactive groups (e.g., methylglyoxal, 4-hydroxynonenal, and glutaconyl-CoA), or metabolites that act as competitive analogs against other metabolites (e.g., deoxyuridine triphosphate and l-2-hydroxyglutarate). Thus, if a metabolic pathway contains a toxic intermediate, then we may be able to induce accumulation and poison a cancer cell by targeting the downstream enzyme. Furthermore, this poisoning may be cancer cell selective if this pathway is overactive in a cancer cell relative to a nontransformed cell. We describe this concept as illustrated in selenocysteine metabolism and other pathways and discuss future directions in exploiting toxic metabolites to kill cancer cells. [1] Despite increasing global prevalence, the precise pathogenesis and terms for objective diagnosis of neurodegenerative dementias remain controversial, and comprehensive understanding of the disease remains lacking. Here, we conducted metabolomic analysis of serum and saliva obtained from patients with neurodegenerative dementias (n = 10), including Alzheimer's disease, frontotemporal lobe dementia, and Lewy body disease, as well as from age-matched healthy controls (n = 9). Using CE-TOF-MS, six metabolites in serum (β-alanine, creatinine, hydroxyproline, glutamine, iso-citrate, and cytidine) and two in saliva (arginine and tyrosine) were significantly different between dementias and controls. Using multivariate analysis, serum was confirmed as a more efficient biological fluid for diagnosis compared to saliva; additionally, 45 metabolites in total were identified as candidate markers that could discriminate at least one pair of diagnostic groups from the healthy control group. These metabolites possibly provide an objective method for diagnosing dementia-type by multiphase screening. Moreover, diagnostic-type-dependent differences were observed in several tricarboxylic acid cycle compounds detected in serum, indicating that some pathways in glucose metabolism may be altered in dementia patients. This pilot study revealed novel alterations in metabolomic profiles between various neurodegenerative dementias, which would contribute to etiological investigations. [2] Quantitative estimates of intracellular fluxes and measurements of intracellular concentrations were used to evaluate the effect of dissolved oxygen (DO) concentration on CRL 1606 hybridoma cells in batch culture. The estimates of intracellular fluxes were generated by combining material balances with measurements of extracellular metabolite rates of change. Experiments were performed at DO levels of 60% and 1% air saturation, as well as under oxygen-limited conditions. Cell extracts were analyzed to evaluate the effect of DO on the intracellular concentrations of the glutamate dehydrogenase reactants, as well as the redox state of the pyridine nucleotides in the cytosol and mitochondria. The relationship between cell density and pyridine nucleotide redox state was also investigated. Dissolved oxygen concentration had a significant effect on nitrogen metabolism and the flux through glutamate dehydrogenase was found to reverse at low DO, favoring glutamate formation. The NAD in the cytosol and mitochondria was more reduced under low DO conditions while the cytosolic NAD was more oxidized at low DO. Cytosolic NAD was reduced at higher cell densities while the redox states of cytosolic NADP and mitochondrial NAD did not exhibit significant variation with cell density. These results point to the fundamental role of the intracellular oxidation/reduction state in cell physiology and the possibility of controlling physiological processes through modulation of the dissolved oxygen level or the oxidation/reduction potential of the culture. [3] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.2051 mL | 26.0254 mL | 52.0508 mL | |
| 5 mM | 1.0410 mL | 5.2051 mL | 10.4102 mL | |
| 10 mM | 0.5205 mL | 2.6025 mL | 5.2051 mL |