Physicochemical Properties
| Molecular Formula | C36H48N4O8 |
| Molecular Weight | 664.788330078125 |
| Exact Mass | 664.347 |
| CAS # | 874882-92-5 |
| PubChem CID | 45073435 |
| Appearance | Typically exists as solid at room temperature |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 16 |
| Heavy Atom Count | 48 |
| Complexity | 386 |
| Defined Atom Stereocenter Count | 0 |
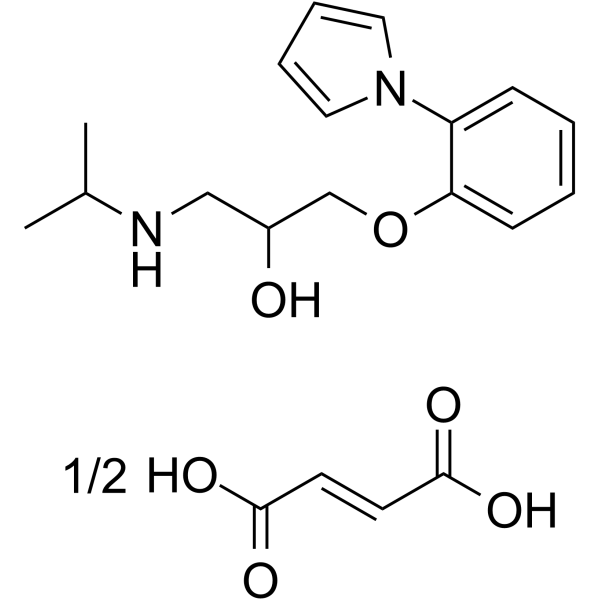
| SMILES | CC(C)NCC(COC1=CC=CC=C1N2C=CC=C2)O.CC(C)NCC(COC1=CC=CC=C1N2C=CC=C2)O.C(=C/C(=O)O)\C(=O)O |
| InChi Key | MCHSBYUSDSJLAQ-WXXKFALUSA-N |
| InChi Code | InChI=1S/2C16H22N2O2.C4H4O4/c2*1-13(2)17-11-14(19)12-20-16-8-4-3-7-15(16)18-9-5-6-10-18;5-3(6)1-2-4(7)8/h2*3-10,13-14,17,19H,11-12H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;;2-1+ |
| Chemical Name | (E)-but-2-enedioic acid;1-(propan-2-ylamino)-3-(2-pyrrol-1-ylphenoxy)propan-2-ol |
| Synonyms | ISAMOLTANE HEMIFUMARATE; 874882-92-5; 1-[(1-Methylethyl)amino]-3-[2-(1H-pyrrol-1-yl)]-propan-2-olhemifumarate; (E)-but-2-enedioic acid;1-(propan-2-ylamino)-3-(2-pyrrol-1-ylphenoxy)propan-2-ol; HMS3267K13; HMS3411N16; HMS3675N16; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-adrenoceptor 8.4 nM (IC50) 5-HT1B Receptor 39 nM (IC50) |
| ln Vitro | In rat brain membranes, isamoltane demonstrates a 27-fold selectivity for the 5-HT1B receptor over the 5-HT1A receptor (IC50=1070 nM)[1]. In rat cortical slices, isamoltane (0.01-10 µM) increases the [3H]-overflow induced by electrical stimulation in a concentration-dependent manner[1]. |
| ln Vivo |
Isamoltane (0.3–30 mg/kg; ip) boosts 5-HT production in cortical tissue while having no effect on the buildup of 5-HTP in the rat hippocampal region. 5-HTP buildup in the striatum is decreased by isamoltane[1]. Isamoltane increased the electrically evoked release of [3H]5-HT from prelabeled rat cortical slices in a manner similar to that of cyanopindolol. While both compounds were similar in potency to methiothepin, they had lower efficacy. Oxprenolol was less potent that both isamoltane and cyanopindolol while propranolol was essentially inactive. The effects of the compounds on 5-HT release appeared to be correlated with their 5-HT1B rather than 5-HT1A activity. In vivo, isamoltane increased 5-HTP accumulation in rat cortex following central decarboxylase inhibition at doses of 1 and 3 mg/kg i.p. At higher doses this effect was gradually diminished. Similar, but less clearcut results were obtained with cyanopindolol and oxprenolol, but propranolol was ineffective. No changes in brain tryptophan levels were associated with the isamoltane-evoked changes in brain 5-HTP levels. In reserpinized animals, isamoltane reduced 5-HTP accumulation even at doses which enhanced accumulation of this metabolite when given alone. The effects of the putative 5-HT1B agonist, m-trifluoromethylphenylpiperazine (TFMPP), the mixed 5-HT autoreceptor agonist/antagonist/beta-adrenoceptor antagonist, pindolol, the 5-HT uptake inhibitor, CGP 6085A and the MAO-A inhibitor, brofaromine, were not antagonized by pretreatment with isamoltane. The possibility that isamoltane and the other beta-adrenoceptor antagonists are antagonists at 5-HT1B receptors and that their effect on 5-HT synthesis in vivo is the net result of their agonist/antagonist effects at 5-HT1A and 5-HT1B receptors is discussed in relation to the potential mechanism of the anxiolytic activity of isamoltane.[1] Isamoltane increased the K(+)-evoked overflow of 3H from 3H-5-HT loaded slices of rat occipital cortex at 0.1 mumol/l, consistent with inhibition of the terminal 5-HT autoreceptor. In vivo, isamoltane significantly increased the concentration of 5-hydroxyindoleacetic acid in hypothalamus and hippocampus indicating an increased 5-HT turnover with a maximal effect at 3 mg/kg s.c. A higher dose produced a less pronounced effect. This effect did not seem to be due to the beta-adrenoceptor blocking action of isamoltane since the beta-adrenoceptor antagonists. (-)-alprenolol, betaxolol or ICI 118.551 had no significant effects on 5-HT turnover at 5 mg/kg s.c. Isamoltane at 3 mg/kg s.c. induced the wet-dog shake response which was blocked by the tryptophan hydroxylase inhibitor p-chlorophenylalanine. In contrast, the same response induced by the 5-HT2 receptor agonist quipazine was not blocked by pretreatment with p-chlorophenylalanine. The wet-dog shakes evoked by isamoltane and quipazine were blocked by ritanserin, which indicates that 5-HT2 receptors are involved in their expression. These observations indicate that isamoltane, by inhibiting the terminal 5-HT autoreceptors, increased the synaptic concentration of 5-HT to a level that induced a behavioural response [2]. |
| Enzyme Assay |
The biochemical and behavioural effects of isamoltane, a beta-adrenoceptor and 5-HT1B receptor antagonist that has higher affinity for 5-HT1B receptors than for 5-HT1A receptors, on 5-HT neurotransmission in the rat brain were examined. In binding experiments isamoltane was found to be about five times more potent as a ligand for the 5-HT1B receptor than for the 5-HT1A receptor (Ki values 21 and 112 nmol/l, respectively). [2] Isamoltane (CGP 361A; (1-(2-(1-pyrrolyl)-phenoxy)-3-isopropylamino-2-propanol hydrochloride), a beta-adrenoceptor ligand (IC50 = 8.4 nmol/l) which has reported activity as an anxiolytic in man was found to be a reasonably active inhibitor of the binding of [125I]ICYP to 5-HT1B recognition sites in rat brain membranes with 27-fold selectivity (IC50 = 39 nmol/l) as compared to the inhibition of binding of [3H]8-OH-DPAT to 5-HT1A receptors (IC50 = 1070 nmol/l). This selectivity was considerably greater than that observed for other beta-adrenoceptor ligands including propranolol (5-HT1A/5-HT1B ratio = 2), oxpenolol (3.5) and cyanopindolol (8.7). The 5-HT1B activity of the compound resided in the (-)-enantiomer. (-)-Isamoltane had weak activity (IC50 3-10 mumol/l) at 5-HT2 and alpha 1-adrenoceptors. The compound was devoid of activity at a number of other central neurotransmitter recognition sites including the 5-HT1C site [1]. |
| Animal Protocol |
Animal/Disease Models: Male SD (Sprague-Dawley) rats (200-300 g)[1] Doses: 0.3, 1, 3, 10, 30 mg/kg Route of Administration: Ip Experimental Results: 38% reduction of 5-HTP accumulation was found with 30 mg/ kg in the striatum. Did not alter the accumulation of 5-HTP in the hippocampus. Increased 5-HT synthesis at 0.3, 1 and 3 mg/kg in cortical tissue. |
| References |
[1]. Waldmeier PC, et, al. Interactions of isamoltane (CGP 361A), an anxiolytic phenoxypropanolamine derivative, with 5-HT1 receptor subtypes in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun; 337(6): 609-20. [2]. Rényi L, et, al. Biochemical and behavioural effects of isamoltane, a beta-adrenoceptor antagonist with affinity for the 5-HT1B receptor of rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1991 Jan;343(1):1-6. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5042 mL | 7.5212 mL | 15.0423 mL | |
| 5 mM | 0.3008 mL | 1.5042 mL | 3.0085 mL | |
| 10 mM | 0.1504 mL | 0.7521 mL | 1.5042 mL |