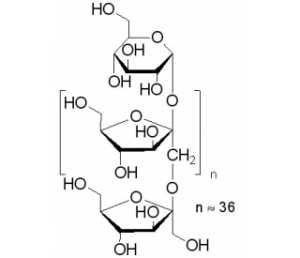
Inulin (Inulin and sodium chloride) is water soluble starch/storage polysaccharide found in the tubers and roots of many plants. Since it is hydrolyzable to fructose, it is classified as a fructosan, which is a non-digestible carbohydrate. Inulin causes (20 g/d and 40 g/d) a significant increase in bifidobacterial counts in feces. Inulin exerts a preferential stimulatory effect on numbers of the health-promoting genus Bifidobacterium, whilst maintaining populations of potential pathogens (Escherichia coli, Clostridium) at relatively low levels. Inulin combined with Bifidobacterium results in more potent inhibition of aberrant crypt foci (ACF) than administration of the two separately, achieving 80% inhibition of small ACF.
Physicochemical Properties
| Molecular Formula | C6NH10N+2O5N+1 | |
| Molecular Weight | 490.411 | |
| Exact Mass | 285.101 | |
| CAS # | 9005-80-5 | |
| Related CAS # |
|
|
| PubChem CID | 254762074 | |
| Appearance | White to off-white solid powder | |
| Density | 1,35 g/cm3 | |
| Boiling Point | 563.5±60.0 °C at 760 mmHg | |
| Melting Point | 176-181ºC | |
| Flash Point | 294.6±32.9 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.665 | |
| Source | Plant/Helianthus tuberosus | |
| LogP | 1.91 | |
| SMILES | C1(O)[C@](CO)(OC[C@]2(O[C@H]3OC(CO)[C@@H](O)C(O)C3O)C(O)C(O)[C@@H](CO)O2)O[C@H](CO)C1O |
|
| InChi Key | UMGSZTYVVMHARA-RYKCJHNISA-N | |
| InChi Code | InChI=1S/C17H30O16/c18-1-5-7(21)12(26)16(3-20,31-5)29-4-17(13(27)8(22)6(2-19)32-17)33-15-11(25)9(23)10(24)14(28)30-15/h5-15,18-28H,1-4H2/t5-,6-,7-,8-,9+,10+,11-,12+,13+,14+,15-,16-,17+/m1/s1 | |
| Chemical Name | (2S,3S,4S,5R,6R)-6-(((2S,3S,4S,5R)-2-((((2R,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-2-yl)oxy)methyl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)oxy)tetrahydro-2H-pyran-2,3,4,5-tetraol | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Cell Assay | Inulin is a water soluble storage polysaccharide and belongs to a group of non-digestible carbohydrates called fructans. Inulin has attained the GRAS status in USA and is extensively available in about 36,000 species of plants, amongst, chicory roots are considered as the richest source of inulin. Commonly, inulin is used as a prebiotic, fat replacer, sugar replacer, texture modifier and for the development of functional foods in order to improve health due to its beneficial role in gastric health. This review provides a deep insight about its production, physicochemical properties, role in combating various kinds of metabolic and diet related diseases and utilization as a functional ingredient in novel product development[1]. | |
| Animal Protocol |
Methods:[4] Thirty apolipoprotein E-deficient (ApoE-/-) mice were randomly divided into three groups. They were fed with a normal diet, a high-fat diet or an inulin+high-fat diet for 16 weeks. The total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) in the three groups were compared. The gross aorta and aortic sinus of mice were stained with oil red O, and the area of atherosclerotic plaque was observed and compared. The diversity and structure of the mouse fecal flora were detected by sequencing the V3-V4 region of the 16S rRNA gene, and the levels of metabolites in mouse feces were assessed by gas chromatography-mass spectrometry. The plasma lipopolysaccharide (LPS) levels and aortic inflammatory factors were measured by multi-index flow cytometry (CBA). Results: [4] ApoE-/- mice fed with the high-fat diet exhibited an increase of approximately 46% in the area of atherosclerotic lesions, and the levels of TC, TG and LDL-C were significantly increased (P < 0.05) compared with levels in the normal diet group. After inulin was added to the high-fat group, the area of atherosclerotic lesions, the level of serum LPS and aortic inflammation were reduced, and the levels of TC, TG and LDL-C were decreased (P < 0.05). Based on 16S rRNA gene detection, we found that the composition of the intestinal microbiota, such as Prevotella, and metabolites, such as L-arginine, changed significantly due to hyperlipidemia, and the dietary inulin intervention partially reversed the relevant changes. Conclusion: [4] Inulin can inhibit the formation of atherosclerotic plaques, which may be related to the changes in lipid metabolism, the composition of the intestinal microbial community and its metabolites, and the inhibition of the expression of related inflammatory factors. Our study identified the relationships among the characteristic intestinal microbiota, metabolites and atherosclerosis, aiming to provide a new direction for future research to delay or treat atherosclerosis by changing the composition and function of the host intestinal microbiota and metabolites. |
|
| References |
[1]. J Nutr.1998 Jan;128(1):11-9; [2]. J Appl Bacteriol.1993 Oct;75(4):373-80; [3]. Carcinogenesis.1998 Feb;19(2):281-5. [4]. Coron Artery Dis. 2024 May 17. doi: 10.1097/MCA.0000000000001377. [5]. Int J Mol Sci. 2024 May 16;25(10):5418. doi: 10.3390/ijms25105418. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (Infinity mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (Infinity mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: Water soluble Solubility in Formulation 4: 27.5 mg/mL (Infinity mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0391 mL | 10.1956 mL | 20.3911 mL | |
| 5 mM | 0.4078 mL | 2.0391 mL | 4.0782 mL | |
| 10 mM | 0.2039 mL | 1.0196 mL | 2.0391 mL |