INCB3344 (INCB-3344 ) is a novel, potent, selective, orally bioavailable small molecule antagonist of the CCR2 receptor with anti-inflammatory activity. It inhibits CCR with IC50 values of 3.8 nM (hCCR2) and 7.8 nM (mCCR2) in antagonism of chemotaxis activity and 5.1 nM (hCCR2) and 9.5 nM (mCCR2) in binding antagonistic activity. Pharmacological studies of CCR2 antagonists and their potential therapeutic utility have been hindered by the inherent lack of rodent cross-reactivity in small molecule antagonists found to date. With nanomolar potency (IC(50) = 10 nM), INCB3344 inhibits CCL2 binding to mouse monocytes in vitro. It also exhibits dose-dependent inhibition of CCL2-mediated functional responses, including ERK phosphorylation and chemotaxis, with a similar potency. INCB3344 is at least 100-fold more selective for CCR2 than other G protein-coupled receptors against which other CC chemokine receptors are present. Because of its high oral bioavailability and systemic exposure in rodents, INCB3344 is suitable for in vivo pharmacological research. INCB3344 treatment results in a dose-dependent inhibition of macrophage influx in a mouse model of delayed-type hypersensitivity. The histopathological examination of tissues from the delayed-type hypersensitivity model indicates that CCR2 inhibition significantly reduces tissue inflammation, pointing to a potential orchestration role for macrophages in immune-based inflammatory responses. The investigation of INCB3344 in inflammatory disease models was prompted by these findings. In mice with experimental autoimmune encephalomyelitis, a model of multiple sclerosis, and in rats with inflammatory arthritis, therapeutic dosage of INCB3344 dramatically ameliorates disease. In conclusion, these findings lend support to the idea of treating chronic inflammatory diseases by targeting this receptor.
Physicochemical Properties
| Molecular Formula | C29H34N3O6F3 | |
| Molecular Weight | 577.59196 | |
| Exact Mass | 577.24 | |
| Elemental Analysis | C, 60.30; H, 5.93; F, 9.87; N, 7.28; O, 16.62 | |
| CAS # | 1262238-11-8 | |
| Related CAS # | INCB3344 R-isomer; cis-INCB3344; 1285539-85-6; 709018-37-1 (isomers) | |
| PubChem CID | 10008367 | |
| Appearance | White to yellow solid powder | |
| LogP | 3.3 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 10 | |
| Rotatable Bond Count | 8 | |
| Heavy Atom Count | 41 | |
| Complexity | 915 | |
| Defined Atom Stereocenter Count | 2 | |
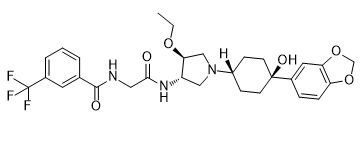
| SMILES | CCO[C@H]1CN(C[C@@H]1NC(=O)CNC(=O)C2=CC(=CC=C2)C(F)(F)F)C3CCC(CC3)(C4=CC5=C(C=C4)OCO5)O |
|
| InChi Key | MZEOSVPWMSEFPW-XYCDVDSTSA-N | |
| InChi Code | InChI=1S/C29H34F3N3O6/c1-2-39-25-16-35(21-8-10-28(38,11-9-21)19-6-7-23-24(13-19)41-17-40-23)15-22(25)34-26(36)14-33-27(37)18-4-3-5-20(12-18)29(30,31)32/h3-7,12-13,21-22,25,38H,2,8-11,14-17H2,1H3,(H,33,37)(H,34,36)/t21?,22-,25-,28?/m0/s1 | |
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | hCCR2 ( IC50 = 5.1 nM ); mCCR2 ( IC50 = 9.5 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Cell Assay | WEHI-274.1, in a 96-well modified Boyden chamber, cells (5×105) in RPMI 1640 (VWR) are loaded into the wells above an 8-μm polycarbonate filter, either with or without different concentrations of INCB3344 in RPMI 1640. A matching 96-well plate containing 30 nM mCCL2—with or without INCB3344—or media is positioned underneath the filter. The sealed chambers are incubated for 45 min at 37°C, 5% CO2. Wright-Giemsa staining is applied to the filters, and microscopy is used to count the number of cells that migrate toward mCCL2 in the bottom chamber. The ability of INCB3344 to antagonize CCR2-mediated chemotaxis is reported as the inhibitor concentration required for IC50 values of specific migration to mCCL2. The total migration less the background migration is known as specific migration. Utilizing mouse MIP-1α as a ligand, an analogous assay is employed to evaluate the effect of INCB3344 on CCR1-mediated chemotaxis of WEHI-274.1 cells. Furthermore, C5a, FMLP, and RANTES are examined in a comparable manner for WEHI-274.1 cell migration when INCB3344 is present. Using mouse MIP-1β as the ligand, murine T cells are employed as the cell system in investigations on the effects of INCB3344 on CCR5-mediated chemotaxis[2]. | |
| Animal Protocol |
|
|
| References |
[1]. Discovery of INCB3344, a potent, selective and orally bioavailable antagonist of human and murine CCR2. Bioorg Med Chem Lett. 2010 Dec 15;20(24):7473-8. [2]. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005 Oct 15;175(8):5370-8. [3]. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice. Hypertension. 2012 Nov;60(5):1207-12. [4]. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J Neurochem. 2008 Jul;106(2):757-69. [5]. Enrichment of Ly6Chi monocytes by multiple GM-CSF injections with HBV vaccine contributes to viral clearance in a HBV mouse model. Hum Vaccin Immunother. 2017 Dec 2;13(12):2872-2882. [6]. CCR2 upregulation in DRG neurons plays a crucial role in gastric hyperalgesia associated with diabetic gastropathy. Mol Pain. 2018 Jan-Dec;14:1744806917751322. [7]. Mcp1 Promotes Macrophage-Dependent Cyst Expansion in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2018 Oct;29(10):2471-2481. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 6 mg/mL (10.39 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: 6 mg/mL (10.39 mM) in 5% DMSO + 95% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. Solubility in Formulation 3: ≥ 2.75 mg/mL (4.76 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 4: 5%DMSO + Corn oil: 5.0mg/ml (8.66mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7313 mL | 8.6567 mL | 17.3133 mL | |
| 5 mM | 0.3463 mL | 1.7313 mL | 3.4627 mL | |
| 10 mM | 0.1731 mL | 0.8657 mL | 1.7313 mL |