INCB054329 is a novel potent and selective inhibitor of Bromodomain and extra-terminal (BET) protein. It that targets Bromodomains 1 (BD1) and BD2 of BRD2, BRD3 and BRD4. INCB054329 inhibited binding of BRD2, BRD3 and BRD4 to an acetylated histone H4 peptide with low nanomolar potency. In myeloma cell lines, treatment with INCB054329 inhibited expression of c-MYC and induced HEXIM1. The majority of myeloma, AML, and lymphoma cell lines tested were growth inhibited by INCB054329 with potencies less than 200 nM. Selectivity was seen when compared with nontransformed cells as the potency for growth inhibition of IL-2 stimulated T-cells from normal donors was greater than 1300 nM. Cell cycle analysis revealed treatment-induced G1 arrest. Furthermore in both AML and lymphoma cell lines, INCB054329 induced apoptosis consistent with increased expression of pro-apoptotic regulators. In vivo, oral administration of INCB054329 inhibited tumor growth in several models of hematologic cancers. In the MM1.S multiple myeloma xenograft model, inhibition of tumor growth was correlated with reduction of c-MYC levels. PK-PD analysis showed c-MYC suppression was associated with an IC50 value of less than 100 nM in vivo. In summary these studies demonstrate that INCB054329 is a potent inhibitor of BET transcriptional regulators in models of hematologic malignancies in vitro and in vivo and support its clinical development for the treatment of cancer.
Physicochemical Properties
| Molecular Formula | C19H16N4O3 |
| Molecular Weight | 348.3553 |
| Exact Mass | 348.122 |
| CAS # | 1628607-64-6 |
| Related CAS # | (R)-INCB054329;1628607-63-5;INCB054329 Racemate;1628607-62-4 |
| PubChem CID | 90410660 |
| Appearance | Light yellow to yellow solid powder |
| LogP | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 26 |
| Complexity | 561 |
| Defined Atom Stereocenter Count | 1 |
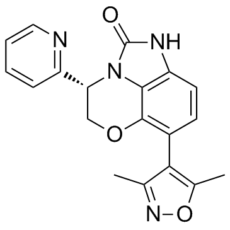
| SMILES | O1C2=C(C3C(C)=NOC=3C)C=CC3=C2N(C(N3)=O)[C@@]([H])(C2=CC=CC=N2)C1 |
| InChi Key | XYLPKCDRAAYATL-OAHLLOKOSA-N |
| InChi Code | InChI=1S/C19H16N4O3/c1-10-16(11(2)26-22-10)12-6-7-14-17-18(12)25-9-15(23(17)19(24)21-14)13-5-3-4-8-20-13/h3-8,15H,9H2,1-2H3,(H,21,24)/t15-/m1/s1 |
| Chemical Name | (11S)-7-(3,5-dimethyl-1,2-oxazol-4-yl)-11-pyridin-2-yl-9-oxa-1,3-diazatricyclo[6.3.1.04,12]dodeca-4(12),5,7-trien-2-one |
| Synonyms | INCB54329; INCB 54329; INCB-54329; INCB054329; INCB-054329; INCB 054329 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | INCB054329 is a bromodomain and extra-terminal motif (BET) inhibitor[1]. INCB054329 inhibits binding of BRD2, BRD3 and BRD4 to an acetylated histone H4 peptide with low nanomolar efficacy. In myeloma cell lines, treatment with INCB054329 inhibited expression of c- MYC and activated HEXIM1. The majority of myeloma, AML, and lymphoma cell lines studied are growth suppressed by INCB054329 with potencies less than 200 nM. Selectivity is demonstrated when compared with nontransformed cells since the potency for growth inhibition of IL-2 activated T- cells from normal donors is greater than 1300 nM. Cell cycle analysis demonstrates treatment-induced G1 arrest. Furthermore in both AML and lymphoma cell lines, INCB054329 promotes apoptosis commensurate with elevated expression of pro-apoptotic regulators[2]. | ||
| ln Vivo | In a number of hematologic cancer models, oral treatment of INCB054329 reduces the growth of tumors. Reduction of c-MYC levels is linked with prevention of tumor growth in the MM1.S multiple myeloma xenograft model. According to PK-PD study, in vivo c-MYC suppression is linked to an IC50 value of less than 100 nM[2]. | ||
| Animal Protocol |
|
||
| References |
[1]. Bromodomain inhibitors and cancer therapy: From structures to applications. Epigenetics. 2017 May 4;12(5):323-339. [2]. Abstract 3523: Discovery of a novel BET inhibitor INCB054329. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.18 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.18 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.18 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8706 mL | 14.3530 mL | 28.7059 mL | |
| 5 mM | 0.5741 mL | 2.8706 mL | 5.7412 mL | |
| 10 mM | 0.2871 mL | 1.4353 mL | 2.8706 mL |