I-CBP112 ( I-CBP-112) is a specific and potent acetyl-lysine competitive protein-protein interaction inhibitor (PPI) with anticancer activity. It inhibits the bromodomain-containing transcription factors CREBBP (CBP) and EP300 with IC50 of 0.142 and 0.625 μM, respectively. It significantly enhances acetylation by p300 at the histone H3K18 and H3K23 sites. I-CBP112 stimulated H3K18ac by ~3-fold, I-CBP112 induced enhances acetylation of these same sites by CBP as well as at H4K5. The EC50's of activation of I-CBP112 on p300- and CBP-mediated H3K18 acetylation are ~2 μM. Exposure of human and mouse leukemic cell lines to I-CBP112 resulted in substantially impaired colony formation and induced cellular differentiation without significant cytotoxicity. I-CBP112 significantly reduced the leukemia-initiating potential of MLL-AF9(+) acute myeloid leukemia cells in a dose-dependent manner in vitro and in vivo. Interestingly, I-CBP112 increased the cytotoxic activity of BET bromodomain inhibitor JQ1 as well as doxorubicin. The synergistic effects of I-CBP112 and current standard therapy (doxorubicin) as well as emerging treatment strategies (BET inhibition) provide new opportunities for combinatorial treatment of leukemia and potentially other cancers.
Physicochemical Properties
| Molecular Formula | C27H36N2O5 | |
| Molecular Weight | 468.59 | |
| Exact Mass | 468.262 | |
| CAS # | 1640282-31-0 | |
| Related CAS # |
|
|
| PubChem CID | 90488984 | |
| Appearance | White to off-white solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 623.5±55.0 °C at 760 mmHg | |
| Flash Point | 330.9±31.5 °C | |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C | |
| Index of Refraction | 1.547 | |
| LogP | 3.29 | |
| Hydrogen Bond Donor Count | 0 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 34 | |
| Complexity | 649 | |
| Defined Atom Stereocenter Count | 1 | |
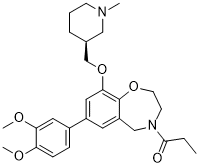
| SMILES | CCC(=O)N1CCOC2=C(C1)C=C(C=C2OC[C@H]3CCCN(C3)C)C4=CC(=C(C=C4)OC)OC |
|
| InChi Key | YKNAKDFZAWQEEO-IBGZPJMESA-N | |
| InChi Code | InChI=1S/C27H36N2O5/c1-5-26(30)29-11-12-33-27-22(17-29)13-21(20-8-9-23(31-3)24(14-20)32-4)15-25(27)34-18-19-7-6-10-28(2)16-19/h8-9,13-15,19H,5-7,10-12,16-18H2,1-4H3/t19-/m0/s1 | |
| Chemical Name | 1-[7-(3,4-dimethoxyphenyl)-9-[[(3S)-1-methylpiperidin-3-yl]methoxy]-3,5-dihydro-2H-1,4-benzoxazepin-4-yl]propan-1-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | I-CBP112 significantly enhanced the acetylation of p300 at histone H3K18 and H3K23 sites. H3K18ac is stimulated by I-CBP112 about three times, and these same sites also experience increased CBP and H4K5 acetylation. With an EC50 of less than 2 μM, I-CBP112 activates p300 and CBP-mediated H3K18 acetylation [1]. I-CBP112 treatment of human and murine leukemia cell lines significantly reduced colony formation and induced cell differentiation without appearing to be harmful. BioMAP primary cell sets exhibit distinct reactions to cytokine and marker protein production upon exposure to I-CBP112 [2]. | ||
| ln Vivo | In both vitro and vivo settings, I-CBP112 dramatically decreased the mLL-AF9+ AML cells' capacity to initiate leukemia in a dose-dependent manner. I-CBP112's synergy with both established standard therapies (doxorubicin) and newer approaches to management (BET inhibition) opens up new avenues for the combined treatment of leukemia and possibly other cancers [2]. | ||
| Animal Protocol |
|
||
| References |
[1]. Modulation of p300/CBP Acetylation of Nucleosomes by Bromodomain LigandI-CBP112. Biochemistry. 2016 Jul 12;55(27):3727-34. [2]. Generation of a Selective Small Molecule Inhibitor of the CBP/p300 Bromodomain for Leukemia Therapy. Cancer Res. 2015 Dec 1;75(23):5106-19. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.34 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1341 mL | 10.6703 mL | 21.3406 mL | |
| 5 mM | 0.4268 mL | 2.1341 mL | 4.2681 mL | |
| 10 mM | 0.2134 mL | 1.0670 mL | 2.1341 mL |