Physicochemical Properties
| Molecular Formula | C23H29IO5 |
| Molecular Weight | 512.38 |
| Exact Mass | 512.105 |
| CAS # | 128719-90-4 |
| PubChem CID | 5311175 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 634.5±55.0 °C at 760 mmHg |
| Melting Point | -144.0 °C |
| Flash Point | 337.5±31.5 °C |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.627 |
| LogP | 4.56 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 29 |
| Complexity | 570 |
| Defined Atom Stereocenter Count | 5 |
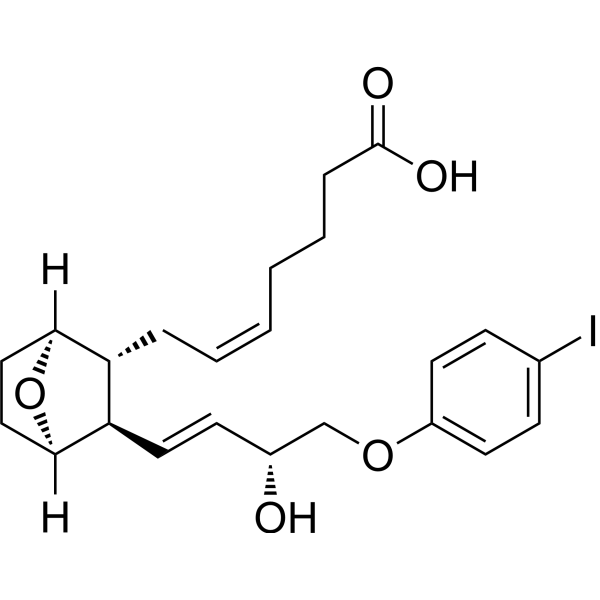
| SMILES | C(=C/C[C@@H]1[C@@H](/C=C/[C@H](COC2=CC=C(C=C2)I)O)C3CCC1O3)/CCCC(=O)O |
| InChi Key | UYFMSCHBODMWON-HBHIRWTLSA-N |
| InChi Code | InChI=1S/C23H29IO5/c24-16-7-10-18(11-8-16)28-15-17(25)9-12-20-19(21-13-14-22(20)29-21)5-3-1-2-4-6-23(26)27/h1,3,7-12,17,19-22,25H,2,4-6,13-15H2,(H,26,27)/b3-1-,12-9+/t17-,19-,20-,21+,22-/m1/s1 |
| Chemical Name | (Z)-7-[(1S,2R,3R,4R)-3-[(E,3R)-3-hydroxy-4-(4-iodophenoxy)but-1-enyl]-7-oxabicyclo[2.2.1]heptan-2-yl]hept-5-enoic acid |
| Synonyms | I-BOP; 128719-90-4; (Z)-7-[(1S,2R,3R,4R)-3-[(E,3R)-3-hydroxy-4-(4-iodophenoxy)but-1-enyl]-7-oxabicyclo[2.2.1]heptan-2-yl]hept-5-enoic acid; 7-[(1S,2R,3R,4R)-3-[(1E,3R)-3-HYDROXY-4-(4-IODOPHENOXY)-1-BUTENYL]-7-OXABICYCLO[2.2.1]HEPT-2-YL]-5Z-; (Z)-7-[(1S,4R,5R,6R)-5-[(E,3R)-3-hydroxy-4-(4-iodophenoxy)but-1-enyl]-7-oxabicyclo[2.2.1]heptan-6-yl]hept-5-enoic acid; (Z)-7-((1S,4R,5R,6R)-5-((E,3R)-3-hydroxy-4-(4-iodophenoxy)but-1-enyl)-7-oxabicyclo(2.2.1)heptan-6-yl)hept-5-enoic acid; GTPL1938; CHEMBL2113346; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Thromboxane A2 receptor (TP) (KD = 0.61 nM) |
| ln Vitro | I-BOP (1-100 nM) activates MAP kinase and stimulates tyrosine phosphorylation, thereby activating the PI3K signaling pathway[1]. At low concentrations, I-BOP (0.1-3 μM) enhances synaptic transmission of hippocampal CA1 neurons by increasing neurotransmitter release; at high concentrations, it inhibits excitatory synaptic transmission of hippocampal CA1 neurons by reducing membrane input resistance[2]. |
| ln Vivo |
1. The effects of the selective thromboxane A2 (TXA2) receptor agonist I-BOP on neuronal excitability and synaptic transmission were studied in the CA1 neurones of rat hippocampal slices by an intracellular recording technique.
2. Superfusion of I-BOP (0.5 μm) resulted in a biphasic change of the excitatory postsynaptic potential (e.p.s.p.), which was blocked by pretreatment with SQ 29548, a specific antagonist of TXA2 receptors. The inhibitory phase of I-BOP on the e.p.s.p. was accompanied by a decrease in neuronal membrane input resistance. 3. The sensitivity of postsynaptic neurones to glutamate receptor agonists, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) or N-methyl-D-aspartate (NMDA), was unchanged by I-BOP (0.5 μm) pretreatment. 4. Bath application of Ba2+ (0.5 mM) prevented both the I-BOP-induced reduction of the neuronal membrane input resistance and the blockade of e.p.s.p. induced by I-BOP. 5. Intracellular dialysis of the hippocampal CA1 neurones with GDP (10 mM) significantly attenuated the I-BOP inhibition of e.p.s.p. and membrane input resistance. Incubation of the slices with either pertussis toxin (PTX, 5 μg ml−1 for 12 h) or cholera toxin (CTX, 5 μg ml-1 for 12 h) did not affect the biphasic action of I-BOP on the e.p.s.p. or the reduction of membrane input resistance induced by I-BOP. 6. Pretreatment of the slices with the protein kinase C (PKC) inhibitor, NPC-15437 (20 μm), abolished the biphasic modulation by I-BOP (0.5 μm) of the e.p.s.p. Intracellular application of a specific PKC inhibitor, PKCI 19–36 (20 μm), completely inhibited the I-BOP reduction of e.p.s.p. The specific cyclic AMP-dependent protein kinase (PKA) inhibitor, Rp-cyclic adenosine 3′,5′-monophosphate (Rp-cyclic AMPS, 25 μm), had no effect on the I-BOP action. 7. In this study we have demonstrated, for the first time, the existence of functional TXA2 receptors in the hippocampus which mediate the effects of a TXA2 agonist on neuronal excitability and synaptic transmission. Activation of the presynaptic TAX2 receptors may stimulate the release of glutamate. Conversely, activation of postsynaptic TXA2 receptors leads to inhibition of synaptic transmission resulting from a decrease in the membrane input resistance of the neurones. The pre- and postsynaptic actions of the TXA2 agonist are both mediated by PTX- and CTX-insensitive G-protein-coupled activation of PKC pathways.[2] |
| Enzyme Assay | Thromboxane A2 (TXA2) interacts with its G-protein coupled receptor, the TP receptor, to produce contraction and proliferation of vascular smooth muscle cells. We have shown previously that proliferation of primary cultures of vascular smooth muscle cells initiated by [1S-(1alpha, 2beta(5Z), 3alpha(1E, 3R), 4alpha]-7-[3-(3-hydroxy-4-(4'-iodophenoxy)-1-butenyl)-7-oxab icyclo-[2.2.1]heptan-2yl]-5'-heptenoic acid (I-BOP), a stable TXA2 mimetic, is mediated by activation of mitogen-activated protein (MAP) kinase. In the present study, we examined further the intracellular mediators involved in TXA2 activation of vascular smooth muscle cells. Transient transfection of the cDNA for the TP receptor into A7r5 vascular smooth muscle cells resulted in expression of TP receptors with a receptor density, Bmax, of 0.7 +/- 0.2 pmol/mg protein and a receptor affinity, Kd, of 0.6 +/- 0.1 nM (N = 7). Mock transfected cells lacked significant receptor expression. In TP receptor transfected cells, I-BOP increased the activation of MAP kinase 2-fold, stimulated tyrosine phosphorylation of cellular proteins of relative molecular mass (Mr) of 140, 85, 60, 56, and 45 kDa, and increased the message for c-jun, a nuclear transcription factor involved in mitogenesis, 2.6-fold. Immunoblot analysis indicated that the 85-kDa protein represented phosphoinositide 3-kinase (PI3-K), while the 60 kDa protein was the TP receptor. The activity of PI3-K was increased 3.5-fold by the addition of I-BOP (0.1 microM). In summary, the present study demonstrated that stimulation of the TP receptor results in tyrosine phosphorylation of the receptor and of PI3-K [1]. |
| Cell Assay |
Western Blot Analysis[1] Cell Types: A7r5.HEL.TP cell Concentration: 1-100 nM Incubation Duration: 24 h Experimental Results: Increased phosphorylated tyrosine. |
| References |
[1]. Tyrosine phosphorylation of phosphatidylinositol 3-kinase and of the thromboxane A2 (TXA2) receptor by the TXA2 mimetic I-BOP in A7r5 cells. Biochem Pharmacol. 1997 Jun 15;53(12):1823-32. [2]. , Thromboxane A2 agonist modulation of excitatory synaptic transmission in the rat hippocampal slice. Br J Pharmacol. 1996 Aug;118(8):2220-7. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9517 mL | 9.7584 mL | 19.5168 mL | |
| 5 mM | 0.3903 mL | 1.9517 mL | 3.9034 mL | |
| 10 mM | 0.1952 mL | 0.9758 mL | 1.9517 mL |