Physicochemical Properties
| Molecular Formula | C30H36O16 |
| Molecular Weight | 652.60 |
| Exact Mass | 652.2 |
| Elemental Analysis | C, 55.21; H, 5.56; O, 39.23 |
| CAS # | 115960-14-0 |
| PubChem CID | 3087722 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.63g/cm3 |
| Boiling Point | 962.7ºC at 760 mmHg |
| Flash Point | 311.1ºC |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.689 |
| LogP | -1.7 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 10 |
| Heavy Atom Count | 46 |
| Complexity | 1040 |
| Defined Atom Stereocenter Count | 10 |
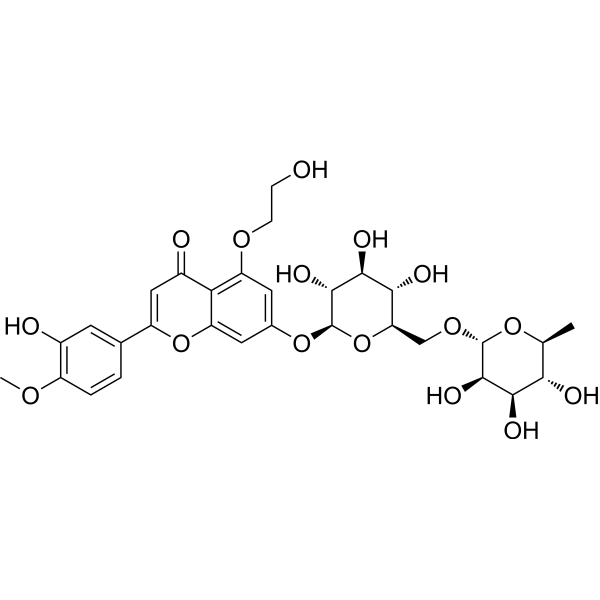
| SMILES | C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H](O2)OC3=CC4=C(C(=C3)OCCO)C(=O)C=C(O4)C5=CC(=C(C=C5)OC)O)O)O)O)O)O)O |
| InChi Key | XYFLWVOTXWXNAM-WTNNCJBMSA-N |
| InChi Code | InChI=1S/C30H36O16/c1-12-23(34)25(36)27(38)29(43-12)42-11-21-24(35)26(37)28(39)30(46-21)44-14-8-19(41-6-5-31)22-16(33)10-18(45-20(22)9-14)13-3-4-17(40-2)15(32)7-13/h3-4,7-10,12,21,23-32,34-39H,5-6,11H2,1-2H3/t12-,21+,23-,24+,25+,26-,27+,28+,29+,30+/m0/s1 |
| Chemical Name | 5-(2-hydroxyethoxy)-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| Synonyms | Hydroxydiosmin; Hidrosmin; 115960-14-0; 4I5K8199OQ; F-117; DTXSID90151237; 5-O-(beta-Hydroxyethyl)diosmin; RefChem:914172; DTXCID2073728; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IL-1β; NOX1; NOX4; The study did not explicitly identify specific molecular targets for Hidrosmin in diabetic nephropathy. Its nephroprotective effects were attributed to suppression of inflammation, oxidative stress, and senescence pathways without direct target binding data. |
| ln Vitro |
Hidrosmin (0.1-1 mM) downregulates cytokine expression and modulates redox homeostasis genes in renal cells exposed to hyperglycemia and/or inflammatory conditions[1]. RT-PCR[1] Cell Line: HK2 cells Concentration: 0.1, 0.3, 1 mM Incubation Time: pretreated for 90 min with hidrosmin before stimulation with either high-glucose (30 mM D-Glucose (HY-B0389)) for 24 h, or a combination of human cytokines interleukin-6 (IL-6, 102 U/mL) and interfero Result: Reduced the gene expression of CC chemokines (CCL2 and CCL5) and proinflammatory cytokines (IL-1β and TNFα) induced by 24 h high-glucose stimulation. Restored the expression of redox balance genes by preventing the prooxidant enzyme NADPH oxidase (NOX1 and NOX4 isoforms) and promoting the expression of antioxidant enzymes Superoxide dismutase-1 (SOD1) and Catalase (CAT) in HK2 cells exposed to high-glucose, as well as to cytokines.
- Anti-Inflammatory and Antioxidant Activity: - Reference [1]: Hidrosmin (10–100 μM) dose-dependently reduced the expression of pro-inflammatory cytokines (TNF-α, IL-6) and oxidative stress markers (iNOS, COX-2) in human renal proximal tubular epithelial cells (HK-2) exposed to high glucose (30 mM) or a cytokine mixture (TNF-α + IFN-γ). This effect was accompanied by decreased nuclear translocation of NF-κB and increased nuclear Nrf2 accumulation. No cytotoxicity was observed at concentrations ≤100 μM (MTT assay showed >90% cell viability). |
| ln Vivo |
Hidrosmin (300 mg/kg, orally administered once daily via feeding for 7 weeks) improves renal damage in diabetic mice [1].
- Renoprotective Efficacy: - Reference [1]: Oral administration of Hidrosmin (300 mg/kg/day) to streptozotocin-induced diabetic ApoE⁻/⁻ mice for 7 weeks significantly reduced albuminuria (albumin-to-creatinine ratio: 47 ± 11% reduction vs. diabetic controls) and improved renal histopathology by attenuating glomerular hypertrophy, mesangial expansion, and tubular fibrosis. Biochemical analysis revealed normalized serum creatinine (1.2 ± 0.1 mg/dL vs. 1.8 ± 0.2 mg/dL in controls) and blood urea nitrogen (25 ± 3 mg/dL vs. 38 ± 5 mg/dL in controls). - Mechanistic Insights: Hidrosmin treatment suppressed renal macrophage infiltration (F4/80-positive cells reduced by 60%) and T-cell accumulation (CD3⁺ cells reduced by 50%), accompanied by downregulation of chemokines (MCP-1, RANTES) and pro-inflammatory cytokines. It also enhanced antioxidant defenses by increasing SOD activity (2.3-fold) and GSH levels (1.8-fold) while reducing MDA content (40% reduction). |
| Cell Assay |
- Renoprotective Efficacy:
- Reference [1]: Oral administration of Hidrosmin (300 mg/kg/day) to streptozotocin-induced diabetic ApoE⁻/⁻ mice for 7 weeks significantly reduced albuminuria (albumin-to-creatinine ratio: 47 ± 11% reduction vs. diabetic controls) and improved renal histopathology by attenuating glomerular hypertrophy, mesangial expansion, and tubular fibrosis. Biochemical analysis revealed normalized serum creatinine (1.2 ± 0.1 mg/dL vs. 1.8 ± 0.2 mg/dL in controls) and blood urea nitrogen (25 ± 3 mg/dL vs. 38 ± 5 mg/dL in controls). - Mechanistic Insights: Hidrosmin treatment suppressed renal macrophage infiltration (F4/80-positive cells reduced by 60%) and T-cell accumulation (CD3⁺ cells reduced by 50%), accompanied by downregulation of chemokines (MCP-1, RANTES) and pro-inflammatory cytokines. It also enhanced antioxidant defenses by increasing SOD activity (2.3-fold) and GSH levels (1.8-fold) while reducing MDA content (40% reduction). |
| Animal Protocol |
Animal/Disease Models:ApoE KO mice (14-16-week-old, induced T1D by streptozotocin injection)[1] Doses: 300 mg/kg/day, dissolved in tap water Route of Administration: Orally through the feeding, once daily for 7 weeks (hidrosmin solution were renewed every 2-3 days) Experimental Results: Did not modify body weight, glycemia, and other biochemical parameters in diabetic mice, except for total cholesterol and low-density lipoprotein (LDL)-cholesterol, which presented a significant reduction when compared with the control group. Ameliorated renal dysfunction by reducing UACR levels. Showed a significant decrease in the urinary KIM-1 levels. - Diabetic Mouse Model: - Reference [1]: Male ApoE⁻/⁻ mice (8 weeks old) were rendered diabetic via intraperitoneal streptozotocin (STZ, 50 mg/kg) for 5 consecutive days. Diabetic mice with fasting blood glucose >250 mg/dL were randomized into two groups: diabetic control (vehicle, 0.5% CMC-Na) and Hidrosmin (300 mg/kg dissolved in 0.5% CMC-Na). Treatment was administered orally once daily for 7 weeks. Urine samples were collected weekly for albumin and creatinine measurements. At sacrifice, kidneys were harvested for histology (PAS staining), immunohistochemistry (F4/80, CD3), and biochemical analysis (SOD, GSH, MDA). |
| Toxicity/Toxicokinetics |
- Safety Profile:
- Reference [1]: No significant adverse effects were observed in Hidrosmin-treated mice, as indicated by normal hematological parameters (WBC, RBC, platelets) and hepatic/renal function markers (ALT, AST, BUN, creatinine). Histological examination of liver and heart tissues showed no signs of toxicity. 3087722 rat LD50 oral >5 gm/kg Drugs of the Future., 12(1015), 1987 3087722 rat LD50 intraperitoneal >5 gm/kg Drugs of the Future., 12(1015), 1987 3087722 rat LD50 intravenous >5 gm/kg Drugs of the Future., 12(1015), 1987 3087722 mouse LD50 oral >5 gm/kg Drugs of the Future., 12(1015), 1987 3087722 mouse LD50 intraperitoneal >5 gm/kg Drugs of the Future., 12(1015), 1987 |
| References |
[1]. Nephroprotective Effects of Synthetic Flavonoid Hidrosmin in Experimental Diabetic Nephropathy. Antioxidants (Basel). 2021 Nov 29;10(12):1920. |
| Additional Infomation |
- Mechanism of Action:
- Reference [1]: Hidrosmin exerts nephroprotection through multi-target mechanisms: (1) suppression of NF-κB-mediated inflammation, (2) activation of Nrf2/HO-1 antioxidant pathway, (3) inhibition of senescence-associated β-galactosidase activity, and (4) attenuation of TGF-β/Smad3-driven fibrosis. These effects collectively mitigate hyperglycemia-induced renal damage. - Therapeutic Potential: - Reference [1]: The study highlights Hidrosmin as a promising adjuvant therapy for diabetic nephropathy, particularly in reducing albuminuria and preserving renal function. Its dual anti-inflammatory and antioxidant properties make it suitable for managing chronic diabetic complications. Hidrosmin is a member of flavonoids and a glycoside. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5323 mL | 7.6617 mL | 15.3233 mL | |
| 5 mM | 0.3065 mL | 1.5323 mL | 3.0647 mL | |
| 10 mM | 0.1532 mL | 0.7662 mL | 1.5323 mL |