Physicochemical Properties
| Molecular Formula | C8H15NO3 |
| Molecular Weight | 173.21 |
| Exact Mass | 173.105 |
| CAS # | 24003-67-6 |
| Related CAS # | Hexanoylglycine-d2;1256842-52-0;Hexanoylglycine-d11 |
| PubChem CID | 99463 |
| Appearance | White to light yellow solid powder |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 387.2±25.0 °C at 760 mmHg |
| Melting Point | 90-92°C |
| Flash Point | 188.0±23.2 °C |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.462 |
| LogP | 0.71 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 12 |
| Complexity | 156 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | UPCKIPHSXMXJOX-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C8H15NO3/c1-2-3-4-5-7(10)9-6-8(11)12/h2-6H2,1H3,(H,9,10)(H,11,12) |
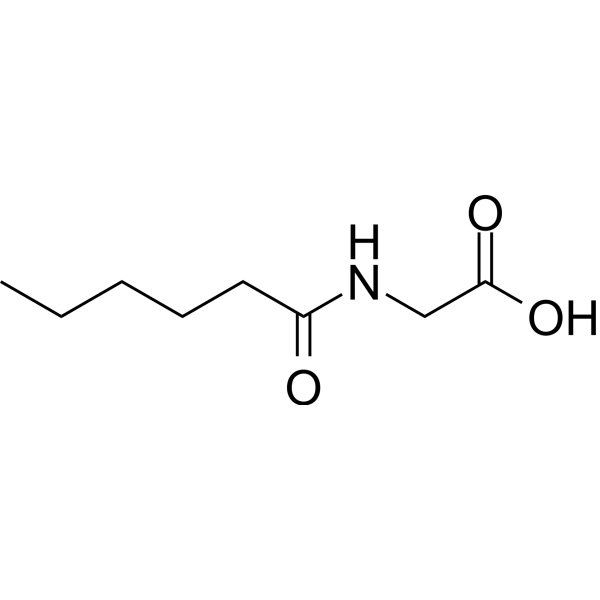
| Chemical Name | 2-(hexanoylamino)acetic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Endogenous metabolites are those that the Kyoto Encyclopedia of Genes and Genomes has identified as products or substrates of the approximately 1900 metabolic enzymes that are encoded in human genome. Numerous of these metabolites have been shown to have harmful effects, as evidenced by the body of literature [1]. |
| References |
[1]. Endogenous toxic metabolites and implications in cancer therapy. Oncogene. 2020 Aug;39(35):5709-5720. [2]. Ethylmalonic and methylsuccinic aciduria in ethylmalonic encephalopathy arise from abnormal isoleucine metabolism. Metabolism. 1998 Jul;47(7):836-9. |
| Additional Infomation |
N-hexanoylglycine is an N-acylglycine in which the acyl group is specified as hexanoyl. It has a role as a metabolite. It is a conjugate acid of a N-hexanoylglycinate. Hexanoylglycine has been reported in Drosophila melanogaster with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO: 100 mg/mL (577.33 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (14.43 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (14.43 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (14.43 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.7733 mL | 28.8667 mL | 57.7334 mL | |
| 5 mM | 1.1547 mL | 5.7733 mL | 11.5467 mL | |
| 10 mM | 0.5773 mL | 2.8867 mL | 5.7733 mL |