Physicochemical Properties
| Molecular Formula | C30H48N12O9 |
| Molecular Weight | 720.777125358582 |
| Exact Mass | 720.366 |
| CAS # | 848644-86-0 |
| Related CAS # | HSDVHK-NH2 TFA |
| PubChem CID | 90488969 |
| Appearance | White to off-white solid powder |
| LogP | -6.6 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 23 |
| Heavy Atom Count | 51 |
| Complexity | 1210 |
| Defined Atom Stereocenter Count | 6 |
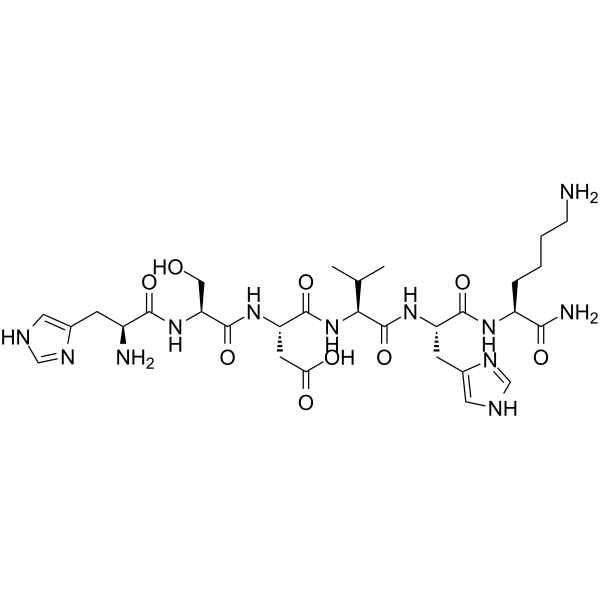
| SMILES | [C@H](C(=O)N[C@H](C(=O)N)CCCCN)(NC(=O)[C@H](C(C)C)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1NC=NC=1)CC1NC=NC=1 |
| InChi Key | FSVRGWKWZIRBPC-KESUXUJOSA-N |
| InChi Code | InChI=1S/C30H48N12O9/c1-15(2)24(30(51)40-20(8-17-11-35-14-37-17)27(48)38-19(25(33)46)5-3-4-6-31)42-28(49)21(9-23(44)45)39-29(50)22(12-43)41-26(47)18(32)7-16-10-34-13-36-16/h10-11,13-15,18-22,24,43H,3-9,12,31-32H2,1-2H3,(H2,33,46)(H,34,36)(H,35,37)(H,38,48)(H,39,50)(H,40,51)(H,41,47)(H,42,49)(H,44,45)/t18-,19-,20-,21-,22-,24-/m0/s1 |
| Chemical Name | (3S)-3-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]-3-hydroxypropanoyl]amino]-4-[[(2S)-1-[[(2S)-1-[[(2S)-1,6-diamino-1-oxohexan-2-yl]amino]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-oxobutanoic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | αvβ3 2.74 nM (IC50) |
| ln Vitro | In comparison to the PBS control group, HSDVHK dramatically reduced the bFGF-induced cell migration[1]. Since HSDVHK-NH2 (P11) is inactive for the complex formation of a denatured version of integrin–vitronectin, its recognition of the Arg-Gly-Asp (RGD)-binding site is site-specific. At an IC50 value of 25.72 nM, HSDVHK-NH2 (P11) exhibits substantial antagonistic activity against the avb3-GRGDSP interaction[2]. By inducing HUVEC cell death by caspase activations, HSDVHK-NH2 (P11) suppresses HUVEC proliferation. This mechanism is associated with enhanced p53 expression[3]. |
| Cell Assay |
Cell Proliferation Assay [3] Cell Types: HUVEC cells. Tested Concentrations: 0.1, 1, 10, and 100 μg/mL. Incubation Duration: 72 h. Experimental Results: Dramatically inhibited HUVEC proliferation on denatured collagen-coated plates in a dose-dependent manner. |
| References |
[1]. High-throughput screening of novel peptide inhibitors of an integrin receptor from the hexapeptide library by using a protein microarray chip. J Biomol Screen. 2004 Dec;9(8):687-94. [2]. Site-specific inhibition of integrin alpha v beta 3-vitronectin association by a ser-asp-val sequence through an Arg-Gly-Asp-binding site of the integrin. Proteomics. 2010 Jan;10(1):72-80. [3]. Pharmacoproteomic analysis of a novel cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol Cell Proteomics. 2011 Aug;10(8):M110.005264. |
Solubility Data
| Solubility (In Vitro) | H2O : 250 mg/mL (346.85 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (138.74 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3874 mL | 6.9369 mL | 13.8739 mL | |
| 5 mM | 0.2775 mL | 1.3874 mL | 2.7748 mL | |
| 10 mM | 0.1387 mL | 0.6937 mL | 1.3874 mL |