Physicochemical Properties
| Molecular Formula | C26H43NO5 |
| Molecular Weight | 449.62 |
| Exact Mass | 467.325 |
| CAS # | 360-65-6 |
| PubChem CID | 3035026 |
| Appearance | Solid powder |
| Density | 1.162 g/cm3 |
| Boiling Point | 655.6ºC at 760 mmHg |
| Flash Point | 350.3ºC |
| Index of Refraction | 1.546 |
| LogP | 3.92 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 32 |
| Complexity | 727 |
| Defined Atom Stereocenter Count | 10 |
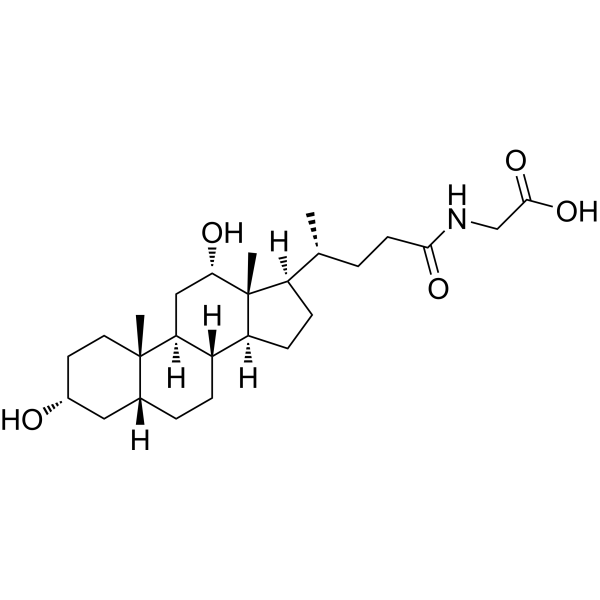
| SMILES | O([H])[C@@]1([H])C([H])([H])[C@@]2([H])[C@@]3(C([H])([H])[H])C([H])([H])C([H])([H])[C@]([H])(C([H])([H])C3([H])C([H])([H])C([H])([H])[C@@]2([H])[C@]2([H])C([H])([H])C([H])([H])[C@]([H])([C@]([H])(C([H])([H])[H])C([H])([H])C([H])([H])C(N([H])C([H])([H])C(=O)O[H])=O)[C@]21C([H])([H])[H])O[H] |
| InChi Key | WVULKSPCQVQLCU-BUXLTGKBSA-N |
| InChi Code | InChI=1S/C26H43NO5/c1-15(4-9-23(30)27-14-24(31)32)19-7-8-20-18-6-5-16-12-17(28)10-11-25(16,2)21(18)13-22(29)26(19,20)3/h15-22,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16-,17-,18+,19-,20+,21+,22+,25+,26-/m1/s1 |
| Chemical Name | 2-[[(4R)-4-[(3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]acetic acid |
| Synonyms | Glycodeoxycholic acid; Glycodeoxycholic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Glycodeoxycholic Acid (200 μM, 24-48 h) induces stemness and chemoresistance of hepatocellular carcinoma cells via the STAT3 signaling pathway[1]. Glycodeoxycholic Acid (50 μM, pretreatment for 1 h) abolishes UCB-induced cytochrome c oxidase inhibition and significantly prevents oxidative stress, metabolic changes, and cell death[2]. |
| ln Vivo | Glycodeoxycholic Acid (11.20 mg/kg, injected into the bile-pancreatic duct) induces acute pancreatitis in macaques[3]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: Huh7, LM3 Concentration: 200 μM Incubation Duration: 24, 48 h Experimental Results: Increased cell viability treated with 5-FU and cisplatin. Western Blot Analysis[1] Cell Types: Huh7, LM3 Concentration: 200 μM Incubation Duration: 24, 48 h Experimental Results: Suppressed the expression of apoptotic genes and increased anti-apoptotic genes. Promoted the expression of Sox2, Sox9, Nanog and CD133. Down-regulated the level of E-cadherin and up-regulated vimentin. Decreased the levels of SOCS2, SOCS5, PTPN1 and PTPN11. |
| Animal Protocol |
Animal/Disease Models:Experimental macaque model[3] Doses: 11.20 mg/kg Route of Administration: injected along the biliopancreatic duct Experimental Results: Increased the levels of Serum amylase and lipase. Elevated Blood pressure and heart rate. |
| References |
[1]. Glycochenodeoxycholic acid induces stemness and chemoresistance via the STAT3 signaling pathway in hepatocellular carcinoma cells. Aging (Albany NY). 2020 Aug 3;12(15):15546-15555. [2]. Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid. J Neurochem. 2010 Jan;112(1):56-65. [3]. Role of glycodeoxycholic acid to induce acute pancreatitis in Macaca nemestrina. J Med Primatol. 2022 Jun;51(3):134-142. doi: 10.1111/jmp.12577. Epub 2022 Mar 20. PMID: 35306662; PMCID: PMC9310849. |
| Additional Infomation |
Glycodeoxycholic acid is a bile acid glycine conjugate of deoxycholic acid. It has a role as a human metabolite. It is functionally related to a deoxycholic acid. It is a conjugate acid of a glycodeoxycholate. Glycodeoxycholic acid has been reported in Streptomyces nigra, Trypanosoma brucei, and Caenorhabditis elegans with data available. A bile salt formed in the liver by conjugation of deoxycholate with glycine, usually as the sodium salt. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and choleretic. |
Solubility Data
| Solubility (In Vitro) | DMSO : 125 mg/mL (278.01 mM; with sonication (<60°C)) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.63 mM)(Saturation unknown) in 10% DMSO 40% PEG300 5% Tween-80 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution, add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix well; then add 50 μL Tween-80 to the above system and mix well; then add 450 μL saline to make up to 1 mL. *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.63 mM)(Saturation unknown) in 10% DMSO 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution, add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD in saline and mix well. *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (4.63 mM)(Saturation unknown) in 10% DMSO 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution, add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL corn oil and mix well. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2241 mL | 11.1205 mL | 22.2410 mL | |
| 5 mM | 0.4448 mL | 2.2241 mL | 4.4482 mL | |
| 10 mM | 0.2224 mL | 1.1121 mL | 2.2241 mL |