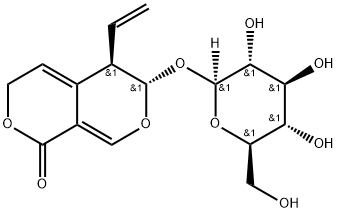
Gentiopicrin is a novel and potent bioactive compound and is naturally occurring iridoid glycoside. Gentiopicrin is an inhibitor of P450 ( IC50 and a Ki of 61 µM and 22.8 µM for CYP2A6 ) and it shows potential activity of anti-inflammation and antioxidation.
Physicochemical Properties
| Molecular Formula | C16H20O9 |
| Molecular Weight | 356.32 |
| Exact Mass | 356.11 |
| CAS # | 20831-76-9 |
| PubChem CID | 88708 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 667.8±55.0 °C at 760 mmHg |
| Melting Point | 191°C |
| Flash Point | 247.1±25.0 °C |
| Vapour Pressure | 0.0±4.6 mmHg at 25°C |
| Index of Refraction | 1.623 |
| LogP | -3.17 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 25 |
| Complexity | 598 |
| Defined Atom Stereocenter Count | 7 |
| SMILES | C=C[C@H]1[C@@H](OC=C2C1=CCOC2=O)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O |
| InChi Key | DUAGQYUORDTXOR-GPQRQXLASA-N |
| InChi Code | InChI=1S/C16H20O9/c1-2-7-8-3-4-22-14(21)9(8)6-23-15(7)25-16-13(20)12(19)11(18)10(5-17)24-16/h2-3,6-7,10-13,15-20H,1,4-5H2/t7-,10-,11-,12+,13-,15+,16+/m1/s1 |
| Chemical Name | (3S,4R)-4-ethenyl-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,6-dihydro-3H-pyrano[3,4-c]pyran-8-one |
| Synonyms | O 673 O-673 O673 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Gentiopiroside has an IC50 of 61 µM and a Ki of 8.12 µM for CYP2A6, respectively, meaning that it inhibits P450 activity. With an IC50 of 1.6 mM, it also marginally suppresses CYP2E1 activity, but not CYP1A2 or CYP3A4 [1]. In a dose-dependent manner, gentiopiroside (12.5, 25, and 50 μM) inhibits the formation of osteoclasts induced by RANKL in mouse bone marrow macrophages (BMM), inhibits the expression of proteins related to osteoclasts, and stops nuclear factor-κB ligand (Receptor activator of RANKL), which activates JNK and NF-κB. Additionally, RANKL-induced bone resorption is inhibited by gentianopicroside (50 μM) [3]. |
| ln Vivo | In mice, gentianopicroside (20, 40, and 80 mg/kg) significantly decreased the index of stomach ulcers. Additionally, gentiopicroside (20, 40, and 80 mg/kg) markedly decreased HSP-70, TNF-α, IL-6, and MDA levels while increasing GSH and SOD activity. Furthermore, gentiopicrin can restore mice's EGF and VEGF levels to normal [2]. |
| References |
[1]. In vitro inhibition and induction of human liver cytochrome P450 enzymes by gentiopicroside: potent effect on CYP2A6. Drug Metab Pharmacokinet. 2013;28(4):339-44. Epub 2013 Feb 19. [2]. Protective effect of gentiopicroside from Gentiana macrophylla Pall. in ethanol-induced gastric mucosal injury in mice. Phytother Res. 2018 Feb;32(2):259-266. [3]. Gentiopicroside inhibits RANKL-induced osteoclastogenesis by regulating NF-κB and JNK signaling pathways. Biomed Pharmacother. 2018 Apr;100:142-146. |
| Additional Infomation |
Gentiopicrin is a glycoside. Gentiopicroside has been reported in Gentiana macrophylla, Gentiana algida, and other organisms with data available. See also: Centaurium erythraea whole (part of). |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~250 mg/mL (~701.62 mM) H2O : ≥ 100 mg/mL (~280.65 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (5.84 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.84 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (5.84 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (280.65 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8065 mL | 14.0323 mL | 28.0647 mL | |
| 5 mM | 0.5613 mL | 2.8065 mL | 5.6129 mL | |
| 10 mM | 0.2806 mL | 1.4032 mL | 2.8065 mL |