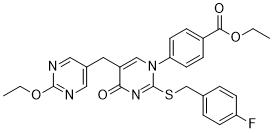
GW-1100 is a novel, potent and selective GPR40 antagonist with a pIC50 of 6.9. GPR40 has been reported to be activated by long-chain fatty acids, such as docosahexaenoic acid (DHA). Unfortunately, there aren't many studies looking into GPR40's functional role in the brain.
Physicochemical Properties
| Molecular Formula | C27H25FN4O4S |
| Molecular Weight | 520.5752 |
| Exact Mass | 520.158 |
| Elemental Analysis | C, 62.30; H, 4.84; F, 3.65; N, 10.76; O, 12.29; S, 6.16 |
| CAS # | 306974-70-9 |
| PubChem CID | 11692123 |
| Appearance | White to off-white solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 690.2±65.0 °C at 760 mmHg |
| Flash Point | 371.2±34.3 °C |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.625 |
| LogP | 4.96 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 37 |
| Complexity | 832 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C1C(CC2=CN=C(N=C2)OCC)=CN(C(SCC3=CC=C(C=C3)F)=N1)C4=CC=C(C(OCC)=O)C=C4 |
| InChi Key | PTPNCCWOTBBVJR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C27H25FN4O4S/c1-3-35-25(34)20-7-11-23(12-8-20)32-16-21(13-19-14-29-26(30-15-19)36-4-2)24(33)31-27(32)37-17-18-5-9-22(28)10-6-18/h5-12,14-16H,3-4,13,17H2,1-2H3 |
| Chemical Name | ethyl 4-[5-[(2-ethoxypyrimidin-5-yl)methyl]-2-[(4-fluorophenyl)methylsulfanyl]-4-oxopyrimidin-1-yl]benzoate |
| Synonyms | GW 1100; GW-1100; GW1100 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GPR40 ( pIC50 = 6.9 ) |
| ln Vitro | GW-1100 (GW1100) dose-inhibitively inhibits the Ca2+ rise mediated by GPR40 stimulated by GW9508 and linoleic acid (pIC50 values of 5.99±0.03 and 5.99±0.06, respectively). GW-1100 at a concentration of 1 μM increased GW9508's There was a significant right shift in the concentration-response curve (pEC50=7.17±0.08 in the absence and pEC50=6.79±0.09 in the presence of 1 μM GW-1100; P<0.05; n=3). At GW-1100 concentrations of 3 μM or higher, a significant decrease in the maximum response was observed and a sustained drift in the pEC50 response [2] was observed. GW-1100 (GW1100) reduces intracellular FFAR1 ligand induction in CHO-K1/bFFAR1 cells and neutrophils CHO-K1/bFFAR1 cells were treated with 10 μM GW1100 or vehicle (0.1% DMSO) for 15 min followed by vehicle , oleic acid, linoleic acid or GW9508 stimulation. GW-1100 was significantly reduced by 300 μM oleic acid (AUC) (60-150 s), p<0.05), 100 μM linoleic acid (AUC (60-150 s), p<0.05) and 10 μM GW9508 (AUC (60-150 s), p<0.05)[3]. |
| ln Vivo | Intracerebroventricular injection of DHA (50 μg) and GW9508 (1.0 μg), a GPR40-selective hemostatic agent, significantly reduced mechanical allodynia and thermal hyperalgesia on day 7 after CFA injection, but not GW on day 1. Intraventricular quiescence with -1100 (10 μg), a GPR40 antagonist, inhibits these effects [4]. |
| Cell Assay | CHO-K1/pcDNA3 or CHO-K1/bFFAR1.In a recording buffer (10 mM HEPES, 140 mM NaCl, 2 mM CaCl2, 21 mM MgCl2, 25 mM KCl, 10 mM glucose, pH 7.4), 1 cell (2×106 cells/2 mL) is loaded with 2.5 μM Fura-2AM fluorescent indicator dye and left for 30 minutes. The cells are then rinsed three times with the recording buffer and put back into the incubator for 10, minutes. Propionic acid (1, 10 and 30 mM), oleic acid (0-500 μM), linoleic acid (0-200 μM), GW9508 (0-100 μM), ionomycin (2 μM), thapsigargin (2 μM), and vehicle (0.1% DMSO) are all incubated with the cells. The fatty acid concentrations utilized in each experiment fall within the range of what healthy, peripartum cows would normally have. In an additional series of tests, after 15 minutes of incubation with either 10 μM GW-1100, 3 minutes with 2 μM U73122, or 15 minutes with vehicle (0.1% DMSO), cells are stimulated with 300 μM oleic acid, 100 μM linoleic acid, or 10 μM GW9508. With a 340/380 nm dual wavelength excitation, cellular fluorescence (Ca2+) is measured at 509 nm emission using an LS55 spectrofluorimeter. Cuvettes are stirred continuously to maintain a temperature of 37°C[3]. |
| Animal Protocol | Mice: Male ddY mice, four weeks of age, are kept in cages at 23–24°C with a 12-hour light–dark cycle (lights on from 8 am to 8 pm), as well as unlimited access to food and drink. Before conducting the von Frey test (with a final concentration of 1% DMSO), the following substances are dissolved in 1% DMSO: DHA (50 µg/mouse), the selective GPR40-agonist GW9508 (1.0-25 µg/mouse), and the GPR40 antagonist GW1100 (1-10 µg/mouse). The dosages of GW9508 are determined by our earlier publication, while the selection of GW-1100 is based on our preliminary experiments and earlier reports. DHA and GW9508 are given intracerebroventricular (i.c.v.) 10 minutes prior to CFA injection, and GW1100 is given via the same route 10 minutes prior to GW9508 injection in a non-anesthetized state. After CFA treatment, mice receive intraperitoneal injections of flavopiridol (5 and 15 nmol/mouse), a cyclin-dependent kinase inhibitor, twice daily (at 9:00 and 19:00) into their left lateral ventricle. |
| References |
[1]. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5'-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol Pharmacol. 2007 Apr;71(4):994-1005. [2]. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006 Jul;148(5):619-28. [3]. Cloning, identification and functional characterization of bovine free fatty acid receptor-1 (FFAR1/GPR40) in neutrophils. PLoS One. 2015 Mar 19;10(3):e0119715. [4]. Hypothalamic GPR40 signaling activated by free long chain fatty acids suppresses CFA-induced inflammatorychronic pain. PLoS One. 2013 Dec 12;8(12):e81563. |
Solubility Data
| Solubility (In Vitro) | DMSO: ~50 mg/mL (~96.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 5 mg/mL (9.60 mM) in 10% DMSO + 40% PEG300 50% PBS (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.80 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 0.06 mg/mL (0.12 mM) in 1% DMSO 99% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9209 mL | 9.6047 mL | 19.2093 mL | |
| 5 mM | 0.3842 mL | 1.9209 mL | 3.8419 mL | |
| 10 mM | 0.1921 mL | 0.9605 mL | 1.9209 mL |