Physicochemical Properties
| Molecular Formula | C23H28CL2N2O2 |
| Molecular Weight | 435.3866 |
| Exact Mass | 364.215 |
| CAS # | 1902123-72-1 |
| Related CAS # | GSK2879552;1401966-69-5 |
| PubChem CID | 66571643 |
| Appearance | White to off-white solid powder |
| LogP | 1.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 27 |
| Complexity | 475 |
| Defined Atom Stereocenter Count | 2 |
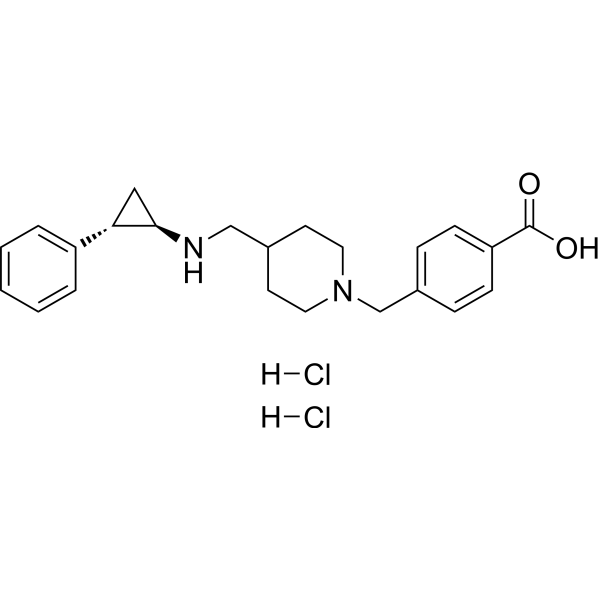
| SMILES | OC(C1C=CC(=CC=1)CN1CCC(CN[C@H]2[C@H](C3C=CC=CC=3)C2)CC1)=O.Cl.Cl |
| InChi Key | LRULVYSBRWUVGR-FCHUYYIVSA-N |
| InChi Code | InChI=1S/C23H28N2O2/c26-23(27)20-8-6-18(7-9-20)16-25-12-10-17(11-13-25)15-24-22-14-21(22)19-4-2-1-3-5-19/h1-9,17,21-22,24H,10-16H2,(H,26,27)/t21-,22+/m0/s1 |
| Chemical Name | 4-[[4-[[[(1R,2S)-2-phenylcyclopropyl]amino]methyl]piperidin-1-yl]methyl]benzoic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | KDM1/LSD1 |
| ln Vitro | GSK2879552 dihydrochloride reduces the characteristics of stem cells, causes sorafenib-resistant cells to differentiate, and inhibits KDM1A histone demethylase activity. In sorafenib-resistant cells, GSK2879552 dihydrochloride downregulates β-catenin signaling activity and inhibits Wnt antagonists' transcription [1]. |
| ln Vivo | In mice containing SCLC xenografts, GSK2879552 dihydrochloride (1.5 mg/kg, po) therapy showed tumor growth reduction [2]. |
| Cell Assay |
Cell Viability Assay[2] Cell Types: 9/28 small cell lung carcinoma (SCLC) lines and 20/29 AML lines. Tested Concentrations: 0-10000 nM. Incubation Duration: 6 days. Experimental Results: Inhibited cell proliferation. RT-PCR[1]. Cell Types: Resistant HCC cells (PLC/PRF/5 and Huh7). Tested Concentrations: 0, 1, 2 μM. Incubation Duration: 24 h. Experimental Results: Displayed decreased mRNA expression levels of stem cell markers, such as Lgr5, Sox9, Nanog and CD90, and elevated mRNA expression levels of differentiation markers Alb and Hnf4. |
| Animal Protocol |
Animal/Disease Models: NCI-H526 and NCI-H1417 xenografts[2]. Doses: 1.5 mg/kg. Route of Administration: PO daily for 25-35 days. Experimental Results: There was 57% and 83% tumor growth inhibition (TGI) in NCI-H526 and NCI-H1417 tumor bearing mice respectively. NCI-H510 and NCI-H69 tumor bearing mice also demonstrated partial TGI (38% and 49% respectively) in response to GSK2879552, while no significant TGI was observed for SHP77 bearing mice. |
| References |
[1]. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett. 2017 Apr 2;398:12-21. [2]. A DNA Hypomethylation Signature Predicts Antitumor Activity of LSD1 Inhibitors in SCLC. Cancer Cell. 2015 Jul 13;28(1):57-69. |
| Additional Infomation |
GSK2879552 is a member of the class of piperidines that is piperidine substituted by (4-carboxyphenyl)methyl and {[(1R,2S)-2-phenylcyclopropyl]amino}methyl groups at positions 1 and 4, respectively. It is a potent and irreversible inhibitor of lysine specific demethylase 1 (LSD1, also known as KDM1A). It was under clinical investigation for the treatment of acute myeloid leukaemia and small cell lung carcinoma. It has a role as an EC 1.14.99.66 (lysine-specific histone demethylase 1A) inhibitor and an antineoplastic agent. It is a member of benzoic acids, a monocarboxylic acid, a member of piperidines, a member of cyclopropanes, a tertiary amino compound, a secondary amino compound and a member of benzenes. LSD1 Inhibitor GSK2879552 is an orally available, irreversible, inhibitor of lysine specific demethylase 1 (LSD1), with potential antineoplastic activity. Upon administration, GSK2879552 binds to and inhibits LSD1, a demethylase that suppresses the expression of target genes by converting the dimethylated form of lysine at position 4 of histone H3 (H3K4) to mono- and unmethylated H3K4. LSD1 inhibition enhances H3K4 methylation and increases the expression of tumor-suppressor genes. This may lead to an inhibition of cell growth in LSD1-overexpressing tumor cells. LSD1, overexpressed in certain tumor cells, plays a key role in tumor cell growth and survival. |
Solubility Data
| Solubility (In Vitro) |
H2O : 100 mg/mL (228.62 mM) DMSO : 31.25 mg/mL (71.44 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.72 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.72 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.72 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 8.33 mg/mL (19.04 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2968 mL | 11.4840 mL | 22.9679 mL | |
| 5 mM | 0.4594 mL | 2.2968 mL | 4.5936 mL | |
| 10 mM | 0.2297 mL | 1.1484 mL | 2.2968 mL |