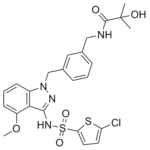
GSK2239633A (GSK-2239633A), a 4-aminoindazole sulfonamide, is a novel and potent antagonist of human CC-chemokine receptor 4 (CCR4) with anti-inflammatory activity. It has a pIC50 of 7.96 ± 0.11 in a focused library of compounds in the primary GTPγS assay, inhibiting the binding of [125I]-TARC to human CCR4. In both the rat (F=62%) and the dog (F=100%), GSK2239633A demonstrated good binding affinity (pIC50=7.2), low lipophilicity (clogP=2.2, chromlogD7.4=2.4), high LE (0.41), high solubility (CLND solubility ≥581µM), and an excellent PK profile. Substitution at N1 is tolerated, according to additional SAR analysis of the pyrazolopyrimidine, which makes it a viable vector for modifying the characteristics and boosting potency in a lead optimization campaign.
Physicochemical Properties
| Molecular Formula | C24H25CLN4O5S2 |
| Molecular Weight | 549.062102079391 |
| Exact Mass | 548.095 |
| Elemental Analysis | C, 52.50; H, 4.59; Cl, 6.46; N, 10.20; O, 14.57; S, 11.68 |
| CAS # | 1240516-71-5 |
| PubChem CID | 46861584 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Index of Refraction | 1.672 |
| LogP | 4.23 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 36 |
| Complexity | 873 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | ClC1=CC=C(S1)S(NC1C2C(=CC=CC=2N(CC2=CC=CC(CNC(C(C)(C)O)=O)=C2)N=1)OC)(=O)=O |
| InChi Key | YTEVTHHGQMUPHC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C24H25ClN4O5S2/c1-24(2,31)23(30)26-13-15-6-4-7-16(12-15)14-29-17-8-5-9-18(34-3)21(17)22(27-29)28-36(32,33)20-11-10-19(25)35-20/h4-12,31H,13-14H2,1-3H3,(H,26,30)(H,27,28) |
| Chemical Name | N-[[3-[[3-[(5-chlorothiophen-2-yl)sulfonylamino]-4-methoxyindazol-1-yl]methyl]phenyl]methyl]-2-hydroxy-2-methylpropanamide |
| Synonyms | GSK-2239633A; GSK 2239633A; GSK2239633A |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | [125I]-TARC-CCR4 ( pIC50 = 7.96 ) |
| ln Vitro | GSK2239633A, an allosteric antagonist of human CCR4. GSK2239633A has a pIC50 of 7.96±0.11 and inhibits the binding of [125I]-TARC to human CCR4. It also has a pA2 of 7.11±0.29[1] that prevents increases in the F-actin content of isolated human CD4+ CCR4+ T-cells that are induced by TARC and the thymus. The effect of GSK2239633A (Compound 3) on CCL17-induced increases in the F-actin content of human CD4+ CCR4+ T cells is measured. The value of pEC50 is 8.79±0.22 [2]. |
| ln Vivo | The rapid, biphasic distribution and slow terminal elimination (t1/2: 13.5 hours) observed in plasma GSK2239633A after intravenous dosing indicate that this medication has low to moderate clearance. Following oral dosing, blood levels of GSK2239633A reach Cmax rapidly (median tmax: 1.0-1.5 hours). Only 16% is the maximum value of estimated GSK2239633A bioavailability, which is low[1]. Bioavailability in rats and beagle dogs is 85% and 97%, respectively, for GSK2239633A (Compound 9) in preclinical animal studies, showing good pharmacokinetic data[3]. |
| Cell Assay | Chemokine-induced increases in the filamentous (F)-actin content of CD4+ CCR4+ T cells are measured in blood drawn from healthy volunteers who have not taken any medication in the previous ten days. Phycoerythrin- and fluorescein isothiocyanate-conjugated anti-CCR4 and anti-human CD4 antibodies are used to stain peripheral blood mononuclear cells (PBMC). The cells are subsequently stimulated with an agonist for 15 seconds after 30 minutes at 37°C incubation with GSK2239633A (1 μM) or vehicle (0.1% DMSO). 3% formaldehyde is added to end the assay. An analysis of the mean fluorescence intensity of 1000 CD4+ CCR4+ cells per sample is conducted using Alexa fluor-647 phalloidin-stained fixed cells. This is given as a percentage of the CD4+ CCR4-cells in the same sample's mean intensity[2]. |
| Animal Protocol | Rats and Dogs: In order to assist in the prediction of the likely human pharmacokinetics using precedented physiological scaling techniques, pharmacokinetics are determined in male Wistar Han rats (277-305 g) or male Sprague Dawley (crl:CD(SD)) rats (277-305 g) and male Beagle dogs (14-16 kg; aged approximately 3-4 years) following single oral and intravenous administration. Compounds (GSK2239633A, for example) are administered intravenously and orally to two rats per compound per route. For intravenous (IV) and oral (PO) administration, nominal doses of 1 mg/kg are utilized, and studies are carried out using standard animal husbandry procedures. Rats are kept in regulated environments and kept in standard holding cages with free access to food and water. All animals have a temporary cannula placed into the vein in their tails to collect serial blood samples. The cannula is placed into a vein that is distinct from the one used for dosing in the animals receiving intravenous doses. After the last dosage on each phase of the study, the dogs are kept in slings for a maximum of two hours. Compounds (such as GSK2239633A) are administered to two male Beagle dogs in a cross-over design either intravenously (bolus, 0.5 mg/kg) using an angiocath or orally (gavage, 1 mg/kg). For the first two hours after the dose, serial blood samples are drawn from the cephalic vein using an angiocath; for the duration of the study, venipuncture is used instead. |
| References |
[1]. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study. BMC Pharmacol Toxicol. 2013 Feb 28;14:14. [2]. Antagonism of human CC-chemokine receptor 4 can be achieved through three distinct binding sites on the receptor. Pharmacol Res Perspect. 2013 Dec;1(2):e00019. [3]. Identification of pyrazolopyrimidine arylsulfonamides as CC-chemokine receptor 4 (CCR4) antagonists. Bioorg Med Chem. 2017 Oct 15;25(20):5327-5340. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8213 mL | 9.1065 mL | 18.2129 mL | |
| 5 mM | 0.3643 mL | 1.8213 mL | 3.6426 mL | |
| 10 mM | 0.1821 mL | 0.9106 mL | 1.8213 mL |