GSK1059865 is a novel, potent and highly selective OX1R antagonist. Treatment with GSK1059865 significantly decreased ethanol drinking in a dose-dependent manner in CIE-exposed mice. GSK1059865, on the other hand, only reduced drinking at the maximum dose when given to mice exposed to air. GSK1059865 had no effect on the amount of sucrose consumed. Therefore, using a highly-selective antagonist to block the OX1R, ORX signaling has a significant impact on high levels of alcohol consumption induced in a dependence paradigm, but has little to no effect on moderate alcohol consumption or sucrose consumption. These findings suggest that treating disorders of compulsive reward seeking, like alcoholism and other addictions with highly elevated motivation, may benefit from targeting the ORX system.
Physicochemical Properties
| Molecular Formula | C20H23BRFN3O2 | |
| Molecular Weight | 436.317927598953 | |
| Exact Mass | 435.095 | |
| CAS # | 1191044-58-2 | |
| Related CAS # |
|
|
| PubChem CID | 44463491 | |
| Appearance | White to off-white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 575.8±50.0 °C at 760 mmHg | |
| Flash Point | 302.1±30.1 °C | |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C | |
| Index of Refraction | 1.592 | |
| LogP | 4.41 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 27 | |
| Complexity | 498 | |
| Defined Atom Stereocenter Count | 2 | |
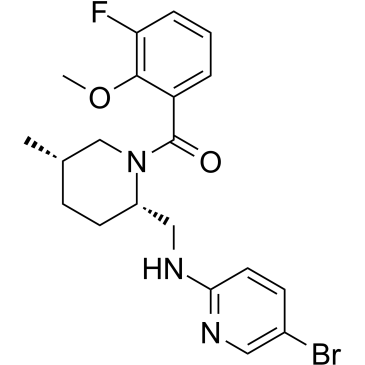
| SMILES | C[C@H]1CC[C@H](N(C1)C(=O)C2=C(C(=CC=C2)F)OC)CNC3=NC=C(C=C3)Br |
|
| InChi Key | TWCRHJLMMAYSTE-ZFWWWQNUSA-N | |
| InChi Code | InChI=1S/C20H23BrFN3O2/c1-13-6-8-15(11-24-18-9-7-14(21)10-23-18)25(12-13)20(26)16-4-3-5-17(22)19(16)27-2/h3-5,7,9-10,13,15H,6,8,11-12H2,1-2H3,(H,23,24)/t13-,15-/m0/s1 | |
| Chemical Name | [(2S,5S)-2-[[(5-bromopyridin-2-yl)amino]methyl]-5-methylpiperidin-1-yl]-(3-fluoro-2-methoxyphenyl)methanone | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Orexin 1 receptor |
| ln Vivo | GSK1059865 treatment dramatically reduces ethanol consumption in CIE-exposed mice in a dose-dependent manner. On the other hand, GSK1059865 only reduces drinking at the maximum dose when given to mice exposed to air. GSK1059865 has no effect on the consumption of sucrose[1]. Non-surmountable antagonism is produced by GSK1059865 (0.3 nM–10 nM), which also causes a dose-dependent rightward shift of the OXA EC50 and a concurrent reduction in the agonist maximal response. The calculated pKB value is 8.77±0.12 for GSK1059865. GSK1059865 (0.1-3.3 μM) generates a classical surmountable profile with a parallel rightward shift of the OXA EC50 without lowering the agonist maximal response[2]. The administration of GSK1059865 intraperitoneally results in a region-specific suppression of the relative cerebral blood volume response induced by yohimbine. In various brain regions, the administration of GSK1059865 alone results in a modest relative increase in cerebral blood volume. Animals pretreated with GSK1059865 have baseline mean arterial blood pressure values that are marginally higher than controls[3]. |
| Animal Protocol |
Rats: GSK1059865 is given to rats by gavage at doses of 10 and 30 mg/kg after being dissolved in 0.5% HPMC (w/v) in distilled water. One hour is allowed before having access to extremely appetizing food for the drug or vehicle[2]. Mice: Mice are injected intraperitoneally (0.01 ml/g body weight) with vehicle (saline) 30 minutes prior to ethanol consumption during baseline and the first five test cycles after chronic intermittent ethanol (or air) exposure. Mice are given either vehicle or GSK1059865 (10, 25, 50 mg/kg) on test cycles 6 and 7. After that, they are allowed to choose between ethanol (15 percent v/v) in Test 6 and sucrose (5% w/v) in Test 7, or water. Using TWEEN 80 (0.5 % v/v) as the vehicle, GSK1059865 is dissolved in salted water[1]. |
| References |
[1]. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016 Apr 1;1636:74-80. [2]. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology. 2012 Aug;37(9):1999-2011. [3]. Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology. 2013 Oct;38(11):2120-30. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 3.33 mg/mL (7.63 mM) in 30 % SBE-β-CD (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. Solubility in Formulation 2: 5 mg/mL (11.46 mM) in 30% PEG300 70% (10% HP-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2919 mL | 11.4595 mL | 22.9190 mL | |
| 5 mM | 0.4584 mL | 2.2919 mL | 4.5838 mL | |
| 10 mM | 0.2292 mL | 1.1459 mL | 2.2919 mL |