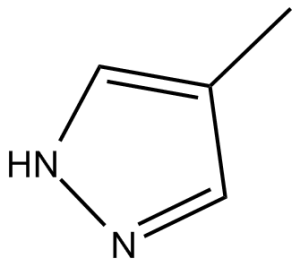
Fomepizole (also known as 4-methylpyrazole, Antizol, Antizol-Vet) is a competitive inhibitor of alcohol dehydrogenase, an enzyme that catalyzes the initial steps in the metabolism of ethylene glycol,ethanol and methanol to their toxic metabolites. Fomepizole is an ADH (alcohol dehydrogenase) inhibitor. It can be used as an antidote in methanol and ethylene glycol poisoning. Fomepizole is used for the treatment of ethylene glycol and methanol poisonings in adults. Fomepizole is a competitive antagonist of alcohol dehydrogenase with a binding affinity >8000 times that of ethanol.
Physicochemical Properties
| Molecular Formula | C4H6N2 | |
| Molecular Weight | 82.1 | |
| Exact Mass | 82.053 | |
| CAS # | 7554-65-6 | |
| Related CAS # | Fomepizole hydrochloride;56010-88-9 | |
| PubChem CID | 3406 | |
| Appearance | Off-white to light yellow <13°C powder,>13°C liquid | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 243.6±0.0 °C at 760 mmHg | |
| Melting Point | 13°C | |
| Flash Point | 96.1±0.0 °C | |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C | |
| Index of Refraction | 1.523 | |
| LogP | 0.78 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 1 | |
| Rotatable Bond Count | 0 | |
| Heavy Atom Count | 6 | |
| Complexity | 44.8 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | N1([H])C([H])=C(C([H])=N1)C([H])([H])[H] |
|
| InChi Key | RIKMMFOAQPJVMX-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C4H6N2/c1-4-2-5-6-3-4/h2-3H,1H3,(H,5,6) | |
| Chemical Name | 4-methyl-1H-pyrazole | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
|
|
| ln Vivo | Fomepizole (4-Methylpyrazole; 25 mg/kg; IP) pretreatment prolongs the neurobehavioral toxicity of ethanol in CD-1 mice[4]. | |
| Animal Protocol |
Animal/Disease Models: Male CD-1 mice weighing 18-25 g[4] Doses: 25 mg/kg Route of Administration: IP; single dose Experimental Results: diminished the dose of ethanol (1-5 g/kg; IP) at which 50% of the animals failed a particular outcome test (toxic dose 50; TD50). |
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Rapid and complete In healthy volunteers, only 1-3.5% of the administered dose of Antizol® (7-20 mg/kg oral and IV) was excreted unchanged in the urine, indicating that metabolism is the major route of elimination. In humans, the primary metabolite of Antizol® is 4-carboxypyrazole (approximately 80-85% of administered dose), which is excreted in the urine. The metabolites of Antizol® are excreted renally. 0.6 to 1.02 L/kg Metabolism / Metabolites Primarily hepatic. the primary metabolite is 4-carboxypyrazole (approximately 80 to 85% of an administered dose). Minor metabolites include 4-hydroxymethylpyrazole and the N -glucuronide conjugates of 4-carboxypyrazole and 4-hydroxymethylpyrazole. Biological Half-Life The plasma half-life of Antizol varies with dose, even in patients with normal renal function, and has not been calculated. |
|
| References |
[1]. Casavant MJ. Fomepizole in the treatment of poisoning. Pediatrics. 2001 Jan;107(1):170. [2]. Adverse drug events associated with the antidotes for methanol and ethylene glycol poisoning: a comparison of ethanol and fomepizole. Ann Emerg Med. 2009 Apr;53(4):439-450.e10. [3]. Use of fomepizole as an adjunct in the treatment of acetaminophen overdose: a case series. Toxicology Communications. Volume 4, 2020 - Issue 1. [4]. Effects of 4-methylpyrazole on ethanol neurobehavioral toxicity in CD-1 mice. Acad Emerg Med. 2004 Aug;11(8):820-6. |
|
| Additional Infomation |
Fomepizole is a member of the class of pyrazoles that is 1H-pyrazole substituted by a methyl group at position 4. It has a role as an antidote, a protective agent and an EC 1.1.1.1 (alcohol dehydrogenase) inhibitor. It derives from a hydride of a 1H-pyrazole. Fomepizole is used as an antidote in confirmed or suspected methanol or ethylene glycol poisoning. Fomepizole is a competitive inhibitor of alcohol dehydrogenase, the enzyme that catalyzes the initial steps in the metabolism of ethylene glycol and methanol to their toxic metabolites. Fomepizole is an Antidote. Fomepizole is a pyrazole with competitive alcohol dehydrogenase inhibitor activity. Fomepizole prevents the metabolism of ethylene glycol and methanol by alcohol dehydrogenase, thereby inhibiting the formation of their toxic metabolites, glycolate and oxalate (from ethylene glycol), and formic acid (from methanol). Fomepizole is indicated for use as an antidote in ethylene glycol and methanol poisoning. (NCI05) A pyrazole and competitive inhibitor of ALCOHOL DEHYDROGENASE that is used for the treatment of poisoning by ETHYLENE GLYCOL or METHANOL. Drug Indication Antizol is indicated as an antidote for ethylene glycol (such as antifreeze) or methanol poisoning, or for use in suspected ethylene glycol or methanol ingestion, either alone or in combination with hemodialysis Mechanism of Action Antizol (fomepizole) is a competitive inhibitor of alcohol dehydrogenase. Alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde. Alcohol dehydrogenase also catalyzes the initial steps in the metabolism of ethylene glycol and methanol to their toxic metabolites. Pharmacodynamics Fomepizole is a competitive inhibitor of alcohol dehydrogenase, the enzyme that catalyzes the initial steps in the metabolism of ethylene glycol and methanol to their toxic metabolites. Ethylene glycol is first metabolized to glycoaldehyde which then undergoes further oxidation to glycolate, glyoxylate, and oxalate. Glycolate and oxalate are primarily responsible for metabolic acidosis and renal damage seen in ethylene glycol toxicity. {01}{03} Methanol is first metabolized to formaldehyde and then undergoes subsequent oxidation via formaldehyde dehydrogenase to become formic acid. It is formic acid that is primarily responsible for the metabolic acidosis and visual disturbances that are associated with methanol poisoning. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (30.45 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (30.45 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (30.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 140 mg/mL (1705.24 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 12.1803 mL | 60.9013 mL | 121.8027 mL | |
| 5 mM | 2.4361 mL | 12.1803 mL | 24.3605 mL | |
| 10 mM | 1.2180 mL | 6.0901 mL | 12.1803 mL |