Physicochemical Properties
| Molecular Formula | C55H77N5O10 |
| Molecular Weight | 968.23 |
| Exact Mass | 967.567 |
| CAS # | 863971-38-4 |
| PubChem CID | 118704820 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 1029.7±65.0 °C at 760 mmHg |
| Flash Point | 576.5±34.3 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.550 |
| LogP | 9.14 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 25 |
| Heavy Atom Count | 70 |
| Complexity | 1700 |
| Defined Atom Stereocenter Count | 9 |
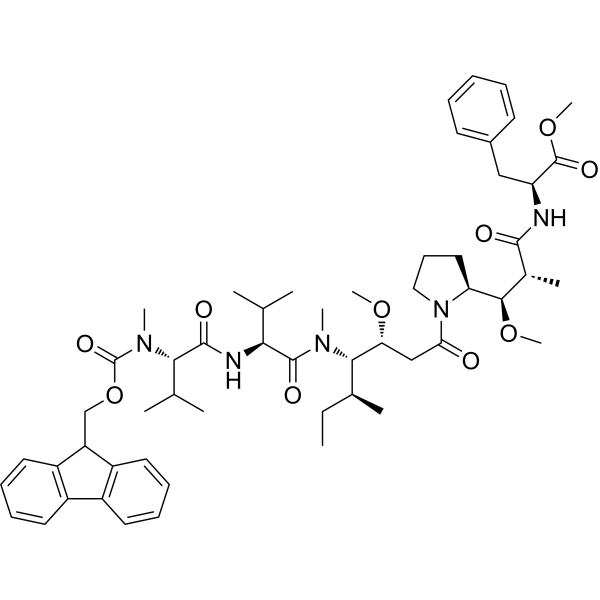
| SMILES | C(C1C2=CC=CC=C2C2C=CC=CC1=2)OC(=O)N(C)[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(=O)OC)CC1C=CC=CC=1)=O |
| InChi Key | MDJMZQUALDTONI-OVPIWJPHSA-N |
| InChi Code | InChI=1S/C55H77N5O10/c1-13-35(6)49(45(67-10)31-46(61)60-29-21-28-44(60)50(68-11)36(7)51(62)56-43(54(65)69-12)30-37-22-15-14-16-23-37)58(8)53(64)47(33(2)3)57-52(63)48(34(4)5)59(9)55(66)70-32-42-40-26-19-17-24-38(40)39-25-18-20-27-41(39)42/h14-20,22-27,33-36,42-45,47-50H,13,21,28-32H2,1-12H3,(H,56,62)(H,57,63)/t35-,36+,43-,44-,45+,47-,48-,49-,50+/m0/s1 |
| Chemical Name | methyl (2S)-2-[[(2R,3R)-3-[(2S)-1-[(3R,4S,5S)-4-[[(2S)-2-[[(2S)-2-[9H-fluoren-9-ylmethoxycarbonyl(methyl)amino]-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methoxy-5-methylheptanoyl]pyrrolidin-2-yl]-3-methoxy-2-methylpropanoyl]amino]-3-phenylpropanoate |
| Synonyms | 863971-38-4; L-Phenylalanine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N-methyl-L-valyl-L-valyl-(3R,4S,5S)-3-methoxy-5-methyl-4-(methylamino)heptanoyl-(alphaR,betaR,2S)-beta-methoxy-alpha-methyl-2-pyrrolidinepropanoyl-, methyl ester; Fmoc-MMAF-OMe; methyl (2S)-2-[[(2R,3R)-3-[(2S)-1-[(3R,4S,5S)-4-[[(2S)-2-[[(2S)-2-[9H-fluoren-9-ylmethoxycarbonyl(methyl)amino]-3-methylbutanoyl]amino]-3-methylbutanoyl]-methylamino]-3-methoxy-5-methylheptanoyl]pyrrolidin-2-yl]-3-methoxy-2-methylpropanoyl]amino]-3-phenylpropanoate; (2R,3R)methyl-2-(N-((2S,3R)-3-((9S)-9-((S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-3-methylbutanamido)-3-methoxy-5,10-dimethyl-4-(methylamino)-8-oxoundecanoyl)pyrrolidin-2-yl)propionamido)-3-methoxy-2-methyl-3-phenylpropanoate; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Tubulin; tubulin polymerization; microtubule |
| ln Vitro |
In vitro sensitivity of endometrial cancer cells to MEDI-547 [1] We next tested the effect of MEDI-547 on in vitro viability of the EphA2 positive Hec-1A and Ishikawa cells. The effect of MEDI-547 was tested at doses ranging from 10 to 50,000 ng/mL. In the Hec-1A cells, compared to controls (either 1C1 or control IgG-mcMMAF), growth was significantly inhibited by MEDI-547 in a dose-dependent manner (Fig. 2A). Similar results were noted with the Ishikawa cells (Fig. S1A). But there was no effect in EPhA2-negative SPEC-2 cells (Fig. S2). Given the decrease in cell viability following treatment, we next asked whether the effects were apoptotic in nature. Treatment with MEDI-547 demonstrated a significant increase in apoptosis compared to treatment with the controls in the Hec-1A (P < 0.05, Fig. 2B), and Ishikawa (P < 0.05, Fig. S1B) cells. |
| ln Vivo |
In vivo tumor growth inhibition of MEDI-547 treated endometrial carcinoma[1] On the basis of the observed effects for cytotoxicity and apoptosis on endometrial cancer cells, we next performed several in vivo experiments using an orthotopic mouse model of uterine cancer to examine the potential therapeutic efficacy of MEDI-547. Mice injected with either Hec-1A or Ishikawa were assigned to one of four groups (n = 10 mice per group): 1) PBS; 2) 1C1, 3 mg/kg once a week; 3) control IgG-mcMMAF, 3 mg/kg once a week; or 4) MEDI-547, 3 mg/kg once a week. Following 5-6 weeks of therapy, the mice were sacrificed and necropsies were performed. Fig. 4B showed that the primary tumor of endometrial carcinoma indeed developed in the uterine cavity and had metastases to ovary. Prolonged MEDI-547 therapy led to a significant reduction in tumor growth in the Hec-1A and Ishikawa models compared with control (PBS), 1C1, or control IgG-mcMMAF (each P < 0.01, Fig. 3A and B, respectively). We next asked whether the MEDI-547 has anti-angiogenic effects in vivo since it also recognizes murine EphA2 (18). To address this question, we used the EphA2 negative SPEC-2 cells (Fig. 1A). The tumor vasculature is known to express higher EphA2 levels compared to normal endothelial cells (18). Although the mean tumor weight of MEDI-547 treated group was lower than the other groups, the difference did not reach statistical significance (Fig. 3C). No obvious signs of toxicity were observed in the treatment groups (i.e. body weight loss)[1]. |
| Cell Assay |
Antibody internalization [1] Procedures for antibody internalization after treatment with MEDI-547 in Hec-1A and Ishikawa cells were performed as described previously. Briefly, viable cells (0.5 × 106) were aliquoted into wells of a 96-well plate in 100 μl of growth media. The cells were centrifuged at 1500 RPM for 5 minutes and labeled with primary antibody drug conjugates by resuspension in 100 μl PBS containing 5 μg of MEDI-547 or control IgG-mcMMAF and incubated for 30 minutes at 4°C. Cells were then washed twice with PBS and cell-surface-bound primary antibody drug conjugates were allowed to internalize by resuspending the cells in 100 μl of growth media and incubation at 37°C / 5% CO2 for 30 minutes or on ice as negative control. Subsequent to internalization, cells were fixed (4% paraformaldehyde, 20 minutes at room temperature), permeabilized (0.5% Triton X-100, 5 minutes at room temperature). Cells were then labeled with secondary AlexaFluor 488 goat anti-human IgG Ab by resuspension in 100 μl PBS + 2% FBS containing 1 ug of secondary antibody and incubated for 30 minutes at 4°C. Cytotoxicity assay [1] The cytotoxic effects of 1C1, control IgG-mcMMAF and MEDI-547 were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide uptake (MTT) assay as described previously. |
| Animal Protocol | The antibody drug conjugate was dosed at weekly 3 mg/kg i.p. injections (14). Therapy experiments were designed using human endometrial cancer cell lines, Hec-1A, Ishikawa, and SPEC-2. Following cell line injection, mice were randomized into four treatment groups: (a) control, 200 μL PBS (i.p., once a week); (b) 1C1, 3 mg/kg in 200 μL PBS (i.p., once a week); (c) control IgG-mcMMAF, 3 mg/kg in 200 μL PBS (i.p., once a week); and (d) MEDI-547, 3 mg/kg in 200 μL PBS (i.p., once a week). Therapy was initiated 2 weeks following cell line injection. Mice were monitored for adverse effects and sacrificed by cervical dislocation 6 to 7 weeks following initiation of treatment. |
| References |
[1]. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin Cancer Res. 2010 May 1;16(9):2562-70. |
| Additional Infomation | Purpose: EphA2 overexpression is frequently observed in endometrial cancers and is predictive of poor clinical outcome. Here, we use an antibody drug conjugate (MEDI-547) composed of a fully human monoclonal antibody against both human and murine EphA2 (1C1) and the tubulin polymerization inhibitor monomethylauristatin F. Experimental design: EphA2 expression was examined in endometrial cancer cell lines by Western blot. Specificity of MEDI-547 was examined by antibody degradation and internalization assays. Viability and apoptosis were investigated in endometrial cancer cell lines and orthotopic tumor models. Results: EphA2 was expressed in the Hec-1A and Ishikawa cells but was absent in the SPEC-2 cells. Antibody degradation and internalization assays showed that the antibody drug conjugate decreased EphA2 protein levels and was internalized in EphA2-positive cells (Hec-1A and Ishikawa). Moreover, in vitro cytotoxicity and apoptosis assays showed that the antibody drug conjugate decreased viability and increased apoptosis of Hec-1A and Ishikawa cells. In vivo therapy experiments in mouse orthotopic models with this antibody drug conjugate resulted in 86% to 88% growth inhibition (P < 0.001) in the orthotopic Hec-1A and Ishikawa models compared with controls. Moreover, the mice treated with this antibody drug conjugate had a lower incidence of distant metastasis compared with controls. The antitumor effects of the therapy were related to decreased proliferation and increased apoptosis of tumor and associated endothelial cells. Conclusions: The preclinical data for endometrial cancer treatment using MEDI-547 show substantial antitumor activity. [1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0328 mL | 5.1641 mL | 10.3281 mL | |
| 5 mM | 0.2066 mL | 1.0328 mL | 2.0656 mL | |
| 10 mM | 0.1033 mL | 0.5164 mL | 1.0328 mL |