Flopropione is a spasmolytic or antispasmodic agent, acting as a 5-HT1A receptor antagonist and also a catechol-o-methyltransferase (COMT) inhibitor. Flopropione does not exhibit Lorentzian relaxation below its T(g) temperature. When the temperature drops below its T(g), flopropione exhibits greater molecular mobility than nifedipine. The entire temperature range of flopropione exhibits an Arrhenius temperature dependence, and the extrapolation of tau (beta) measured above T (g) by dielectric relaxation agreed with tau (beta) measured below T (g) by TAM/MDSC.
Physicochemical Properties
| Molecular Formula | C9H10O4 | |
| Molecular Weight | 182.17 | |
| Exact Mass | 182.058 | |
| Elemental Analysis | C, 59.34; H, 5.53; O, 35.13 | |
| CAS # | 2295-58-1 | |
| Related CAS # |
|
|
| PubChem CID | 3362 | |
| Appearance | Yellow to orange solid powder | |
| Density | 1.372g/cm3 | |
| Boiling Point | 341.7ºC at 760mmHg | |
| Melting Point | 177°C | |
| Flash Point | 174.7ºC | |
| Vapour Pressure | 4E-05mmHg at 25°C | |
| Index of Refraction | 1.618 | |
| LogP | 1.396 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 13 | |
| Complexity | 180 | |
| Defined Atom Stereocenter Count | 0 | |
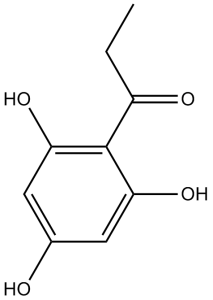
| SMILES | O([H])C1C([H])=C(C([H])=C(C=1C(C([H])([H])C([H])([H])[H])=O)O[H])O[H] |
|
| InChi Key | PTHLEKANMPKYDB-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C9H10O4/c1-2-6(11)9-7(12)3-5(10)4-8(9)13/h3-4,10,12-13H,2H2,1H3 | |
| Chemical Name | 1-(2,4,6-trihydroxyphenyl)propan-1-one | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | COMT; 5-HT1A Receptor | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 2015 Jan 6;21(1):126-37. [2]. Modulation of neurogenesis using d-cycloserine combinations. 2010-08-26. PAT - US2010216805. [3]. Facilitation of expulsion of ureteral stones by addition of α1-blockers to conservative therapy. Scand J Urol Nephrol. 2010 Dec;44(6):420-4. |
||
| Additional Infomation | Flopropione is an organic molecular entity. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (13.72 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (13.72 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (13.72 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.4894 mL | 27.4469 mL | 54.8938 mL | |
| 5 mM | 1.0979 mL | 5.4894 mL | 10.9788 mL | |
| 10 mM | 0.5489 mL | 2.7447 mL | 5.4894 mL |