Physicochemical Properties
| Molecular Formula | C18H18FN5OS |
| Molecular Weight | 371.4318 |
| Exact Mass | 371.12 |
| Elemental Analysis | C, 58.21; H, 4.88; F, 5.11; N, 18.86; O, 4.31; S, 8.63 |
| CAS # | 932737-65-0 |
| PubChem CID | 16660135 |
| Appearance | White to off-white solid powder |
| Melting Point | 174 °C |
| LogP | 1.45 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 26 |
| Complexity | 475 |
| Defined Atom Stereocenter Count | 0 |
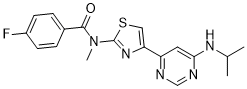
| SMILES | O=C(N(C)C1SC=C(C2C=C(NC(C)C)N=CN=2)N=1)C1C=CC(F)=CC=1 |
| InChi Key | WIVGIKIKQHUFOD-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C18H18FN5OS/c1-11(2)22-16-8-14(20-10-21-16)15-9-26-18(23-15)24(3)17(25)12-4-6-13(19)7-5-12/h4-11H,1-3H3,(H,20,21,22) |
| Chemical Name | 4-fluoro-N-methyl-N-[4-[6-(propan-2-ylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]benzamide |
| Synonyms | FITM |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | mGlu1 ( Ki = 2.5 nM ) |
| ln Vitro | FITM slides into the long, narrow pocket with precision. Most of the ligand-receptor interactions are hydrophobic with the exception of the contacts of the pyrimidine-amine group with the T815 7.38 side chain. The mGlu1 binding pocket for FITM largely corresponds to mutagenic data for the common allosteric site in mGlus and likely extends to other class C GPCRs. FITM, which exhibits superior selectivity and affinity for mGlu1 over mGlu5[1]. During molecular dynamics simulations, FITM has a high hydrogen bond occupancy with Tyr805 and Thr815 in dimers A and B of mGlu1. Dynamic hydrogen bonds are formed by the nitrogen and hydrogen atoms of FITM with the oxygen and hydrogen atoms of Thr815 and Tyr805, respectively. It suggests that FITM and allosteric sites interact strongly through attraction[2]. |
| ln Vivo | The input function is unaffected by the pretreatment of rats with unlabeled FITM (1 mg/kg), which occupies more than 99% of the mGluR1 binding site of 18F-FITM. The Kd (nM) and Bmax (pmol/mL) obtained by the Scatchard analyses with the multidose ligand assays are 2.1 and 36.3, respectively, for the thalamus; 2.1 and 27.5, respectively, for the hippocampus; 1.5 and 22.2, respectively, for the striatum; and 1.5 and 20.5, respectively, for the cingulate cortex with a high confidence[3]. 18F-FITM shows excellent pharmacokinetics, namely the dense and specific accumulation in mGlu1-positive melanomas versus mGlu1-negative hepatoma and normal tissues. In addition, levels of mGlu1 protein expression in melanomas and melanoma metastases were correlated with the accumulation levels of radioactivity[4]. |
| Animal Protocol | Rats: Different doses of unlabeled FITM (0, 1, 5, or 30 μg/kg or 1 mg/kg) are administered to Sprague-Dawley rats prior to a bolus injection of 18F-FITM (17–18 MBq, 30–40 pmol, 0.1 mL). Acquired were estimates of the equilibrium state and BPND [3]. |
| References |
[1]. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science. 2014 Apr 4;344(6179):58-64. [2]. Investigation of allosteric modulation mechanism of metabotropic glutamate receptor 1 by molecular dynamics simulations, free energy and weak interaction analysis. Sci Rep. 2016 Feb 18;6:21763. [3]. In vivo measurement of the affinity and density of metabotropic glutamate receptor subtype 1 in rat brain using 18F-FITM in small-animal PET. J Nucl Med. 2012 Oct;53(10):1601-7. [4]. Molecular imaging of ectopic metabotropic glutamate 1 receptor in melanoma with a positron emission tomography radioprobe (18) F-FITM. Int J Cancer. 2014 Oct 15;135(8):1852-9. |
Solubility Data
| Solubility (In Vitro) | DMSO: ~100 mg/mL (~269.2 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.73 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.73 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6923 mL | 13.4615 mL | 26.9230 mL | |
| 5 mM | 0.5385 mL | 2.6923 mL | 5.3846 mL | |
| 10 mM | 0.2692 mL | 1.3461 mL | 2.6923 mL |