Physicochemical Properties
| Molecular Formula | C45H65N13O11S2.XC2HF3O2 |
| Molecular Weight | 1028.21 (free base) |
| Exact Mass | 1027.44 |
| Elemental Analysis | C, 52.57; H, 6.37; N, 17.71; O, 17.12; S, 6.24 |
| Related CAS # | F992;162277-99-8 |
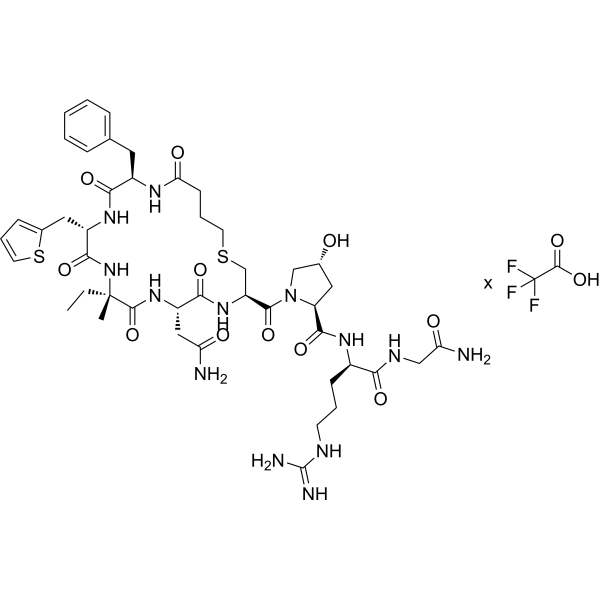
| Sequence | Butanoic acid-{D-Phe}-{Thi}-{α-Me-Abu}-Asn-Cys-{Hyp}-{D-Arg}-Gly-NH2 (Carba sulfide bridge:butanoic acid-1 to Cys-6) |
| SequenceShortening | [D-Phe2, Thi3, alpha-Me-Abu4, Hyp7, D-Arg8]dC1-vasopressin |
| Appearance | White to off-white solid |
| LogP | -2.7 |
| InChi Key | QWIZXUKSCAJBBY-NDJSDKDCSA-N |
| InChi Code | 1S/C45H65N13O11S2/c1-3-45(2)43(69)56-31(21-34(46)60)39(65)55-32(42(68)58-23-26(59)19-33(58)41(67)53-28(13-7-15-50-44(48)49)37(63)51-22-35(47)61)24-70-16-9-14-36(62)52-29(18-25-10-5-4-6-11-25)38(64)54-30(40(66)57-45)20-27-12-8-17-71-27/h4-6,8,10-12,17,26,28-33,59H,3,7,9,13-16,18-24H2,1-2H3,(H2,46,60)(H2,47,61)(H,51,63)(H,52,62)(H,53,67)(H,54,64)(H,55,65)(H,56,69)(H,57,66)(H4,48,49,50)/t26-,28-,29-,30+,31+,32+,33+,45+/m1/s1 |
| Chemical Name | (2S,4R)-N-[(2R)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]-1-[(3R,6S,9S,12S,15R)-6-(2-amino-2-oxoethyl)-15-benzyl-9-ethyl-9-methyl-5,8,11,14,17-pentaoxo-12-(thiophen-2-ylmethyl)-1-thia-4,7,10,13,16-pentazacycloicosane-3-carbonyl]-4-hydroxypyrrolidine-2-carboxamide |
| Synonyms | F992; N39Q2H8V0B; UNII-N39Q2H8V0B; F-992 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | vasopressin receptor |
| ln Vivo | The target dose was found to be 4.0 microg as this dose fulfilled the criteria regarding antidiuretic effect, consequently 8.0 microg was administered to all subjects in the safety study. After infusion of 4.0 and 8.0 microg, the median half-lives of elimination were 4.72 (range 3.99-6.53) h and 3.85 (range 3.04-11.08) h, respectively. The plasma clearance and the volume of distribution at steady state were estimated to be 0.88 (SD 0.24) ml x min(-1) x kg(-1) and 326 (SD 68) ml x kg(-1)] after infusion of 4 microg. After the highest dose (8 microg), the corresponding estimates were 0.86 (SD 0.32) ml x min(-1) x kg(-1) and 299 (SD 81) ml x kg(-1), respectively. Significantly (P = 0.033) different maximum mean urine osmolalities were produced after infusion of 4.0 and 8.0 microg of F992 (534 (SD 318) vs 732 (SD 189) mOsmol x kg(-1)). The median times to reach these values showed some tendency to be longer for the highest dose, however statistical significance was not reached. No serious adverse events were observed during the study.[1] |
| Animal Protocol | Eight healthy male volunteers participated in this open study consisting of two parts: a dose titration study and a safety study. In the dose titration study ascending doses of F992 were administered to volunteers in pairs in order to find a dose that within 1 h after the infusion, in both subjects, caused a reduction of the urine flow rate to below 5 ml x min(-1) (target dose). Subsequently, this target dose was administered to all volunteers. In the safety study the target dose was doubled and given to all volunteers. On each study occasion, in both study parts, the subjects were orally overhydrated with water. F992 was administered as i.v. infusion approximately 1.5 h after the start of the hydration procedure. Throughout the study days, blood was sampled for determination of plasma concentrations of F992 and for safety evaluation. Urine was collected at intervals in order to estimate flow rate and osmolality.[1] |
| References |
[1]. Pharmacokinetics and antidiuretic effect of a new vasopressin analogue (F992) in overhydrated male volunteers. Eur J Clin Pharmacol. 1999 Jun;55(4):293-8. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |