Physicochemical Properties
| Molecular Formula | C36H42N6O7 |
| Molecular Weight | 670.754688739777 |
| Exact Mass | 670.311 |
| CAS # | 1276123-71-7 |
| Related CAS # | Endomorphin 1;189388-22-5 |
| PubChem CID | 165437298 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 49 |
| Complexity | 1060 |
| Defined Atom Stereocenter Count | 4 |
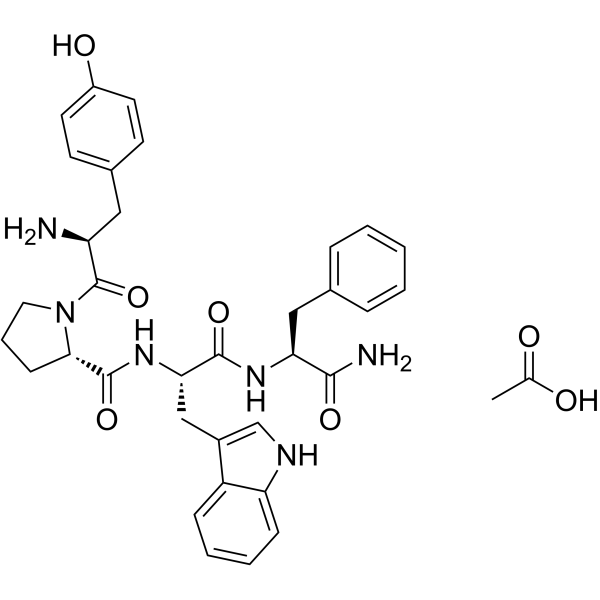
| SMILES | CC(=O)O.C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)N)C(=O)N[C@@H](CC3=CNC4=CC=CC=C43)C(=O)N[C@@H](CC5=CC=CC=C5)C(=O)N |
| InChi Key | ISCQEYDGKZTCMS-YAOJAVRNSA-N |
| InChi Code | InChI=1S/C34H38N6O5.C2H4O2/c35-26(17-22-12-14-24(41)15-13-22)34(45)40-16-6-11-30(40)33(44)39-29(19-23-20-37-27-10-5-4-9-25(23)27)32(43)38-28(31(36)42)18-21-7-2-1-3-8-21;1-2(3)4/h1-5,7-10,12-15,20,26,28-30,37,41H,6,11,16-19,35H2,(H2,36,42)(H,38,43)(H,39,44);1H3,(H,3,4)/t26-,28-,29-,30-;/m0./s1 |
| Chemical Name | acetic acid;(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-N-[(2S)-1-[[(2S)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]pyrrolidine-2-carboxamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | μ Opioid Receptor/MOR 1.11 nM (Ki) |
| ln Vitro | With a pIC50 value of 8.03 in CHOμ cells, endomorphin 1 acetate suppresses the production of cyclic AMP induced by forskolin (1 μM)[5]. In Caco-2 cells, endomorphin 1 (1–10 μM) acetate stimulates the release of interleukin-8[6]. Adult rat substantia gelatinosa neurons exhibit excitatory transmission inhibition upon exposure to 1 μM acetate of endomorphin 1[7]. |
| ln Vivo | Endomorphin 1 (icv) acetate has an ED50 value of 6.16 nM in mice, indicating antinociceptive characteristics[2]. By suppressing the inflammatory response, endomorphin 1 (50 μg/kg, IV, rats) acetate reduces myocardial ischemia/reperfusion injury (MIRI)[3]. |
| Animal Protocol |
Animal/Disease Models: ICR mice[2]. Doses: 6.16 nM (ED50) Route of Administration: Intracerebroventricularly (icv) injection Experimental Results: Inhibited dose-dependently the tail-flick response. Animal/Disease Models: Rats[3]. Doses: 50 μg/kg Route of Administration: intravenously (iv) following LAD ligation for 25 min, subsequently the LAD was reperfused for 120 min. Experimental Results: Alleviated MIRI by reducing the production of free radicals. Dncreased LDH and CK-MB activities. Increased SOD activity and diminished MDA content. diminished IL-6 and TNF-α plasma content. |
| References |
[1]. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J Pharmacol Exp Ther. 1998 Aug;286(2):1007-13. [2]. Tseng LF. The antinociceptive properties of endomorphin-1 and endomorphin-2 in the mouse. Jpn J Pharmacol. 2002 Jul;89(3):216-20. [3]. Effects of endomorphin-1 postconditioning on myocardial ischemia/reperfusion injury and myocardial cell apoptosis in a rat model. Mol Med Rep. 2016 Oct;14(4):3992-8. [4]. Synthesis and in vitro evaluation of a library of modified endomorphin 1 peptides. Bioorg Med Chem. 2008 Jun 1;16(11):6286-96. [5]. The effects of endomorphin-1 and endomorphin-2 in CHO cells expressing recombinant mu-opioid receptors and SH-SY5Y cells. Br J Pharmacol. 1999 Sep;128(2):472-8. [6]. Endomorphin-1 alters interleukin-8 secretion in Caco-2 cells via a receptor mediated process. Immunol Lett. 2002 Dec 3;84(3):217-21. [7]. Inhibition by endomorphin-1 and endomorphin-2 of excitatory transmission in adult rat substantia gelatinosa neurons. Neuroscience. 2006;139(3):1095-105. |
Solubility Data
| Solubility (In Vitro) | DMSO: 125 mg/mL (186.36 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.10 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.10 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.10 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4909 mL | 7.4543 mL | 14.9087 mL | |
| 5 mM | 0.2982 mL | 1.4909 mL | 2.9817 mL | |
| 10 mM | 0.1491 mL | 0.7454 mL | 1.4909 mL |