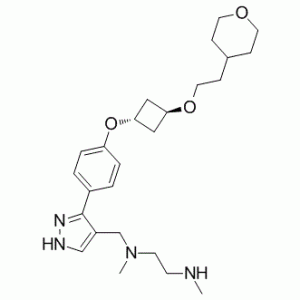
EPZ020411 (EPZ-020411) is a selective PRMT6 (protein arginine methyltransferase) inhibitor with antitumor effects. It inhibits PRMT6 with an IC50 of 10 nM. Overexpression of PRMT6 has been found in several types of cancer, suggesting that inhibition of PRMT6 activity may have therapeutic utility. EPZ020411 shows good bioavailability following subcutaneous dosing in rats making it a suitable tool for in vivo studies. In biochemical assays, EPZ020411 was found to be over 100-fold selective for PRMT6/8/1 compared to other histone methyltransferases, such as PRMT3, PRMT4, PRMT5 and PRMT7. In addition, EPZ020411 treatment led to a dose-dependent decrease in H3R2 methylation, while treatment with its PRMT6-inactive analog did not generate an IC50 at concentrations up to 20 μM. EPZ020411 shows good bioavailability following subcutaneous (SC) dosing in rats making it a suitable tool for in vivo studies.
Physicochemical Properties
| Molecular Formula | C25H40CL2N4O3 | |
| Molecular Weight | 515.52 | |
| Exact Mass | 442.294 | |
| CAS # | 1700663-41-7 | |
| Related CAS # | EPZ020411 hydrochloride;2070015-25-5 | |
| PubChem CID | 91668547 | |
| Appearance | Off-white to yellow ointment | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 619.2±55.0 °C at 760 mmHg | |
| Flash Point | 328.3±31.5 °C | |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C | |
| Index of Refraction | 1.579 | |
| LogP | 1.92 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 12 | |
| Heavy Atom Count | 32 | |
| Complexity | 519 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O(C([H])([H])C([H])([H])C1([H])C([H])([H])C([H])([H])OC([H])([H])C1([H])[H])C1([H])C([H])([H])C([H])(C1([H])[H])OC1C([H])=C([H])C(C2=C(C([H])=NN2[H])C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])N([H])C([H])([H])[H])=C([H])C=1[H] |
|
| InChi Key | QMDKVNSQXPVCRD-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C25H38N4O3/c1-26-10-11-29(2)18-21-17-27-28-25(21)20-3-5-22(6-4-20)32-24-15-23(16-24)31-14-9-19-7-12-30-13-8-19/h3-6,17,19,23-24,26H,7-16,18H2,1-2H3,(H,27,28) | |
| Chemical Name | N1,N2-dimethyl-N1-((3-(4-((1r,3r)-3-(2-(tetrahydro-2H-pyran-4-yl)ethoxy)cyclobutoxy)phenyl)-1H-pyrazol-4-yl)methyl)ethane-1,2-diamine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In A375 cells, EPZ020411 (0–20 μM; 24 h) reduces H3R2 methylation[1]. Hair cell survival is increased and neomycin- and cisplatin-induced cell apoptosis is reduced by EPZ020411 (20–40 μM; 6 h)[2]. |
| ln Vivo | With an acute ototoxicity model, EPZ020411 (10 mg/kg; ip once) lessens the hearing loss caused by neomycin and cisplatin in C57BL/6J wild-type mice[2]. Rats' 1.19 Pharmacokinetic EPZ020411 Parameters[1]. Rats IV 1 mg/kg Rats SC 5 mg/kg CL (mL/min/kg) 19.7±1.0 Vss (L/kg) 11.1±1.6 t1/2 (h) 8.54±1.43 9.19±1.60 tmax (h) 0.444 Cmax (ng/mL) 844±306 AUC0-τ (h·ng/mL) 745±34 2456±135 AUC0-inf (h·ng/mL) 846±45 2775±181 F (%) 65.6±4.3 |
| Cell Assay |
Western Blot Analysis[1] Cell Types: A375 cells Tested Concentrations: 0-20 μM Incubation Duration: 24 hrs (hours) Experimental Results: Dose-dependently diminished H3R2 methylation in A375 cells with an IC50 of 0.634 μM. Apoptosis Analysis[2] Cell Types: Cultured cochleae cells Tested Concentrations: 20-40 μM Incubation Duration: 6 hrs (hours) Experimental Results: Suppressed the apoptotic cascade induced by aminoglycosides and also inhibited cisplatin-induced apoptosis in the hair cells of the cochlear implants after pretreatment deposed. Also decreased hair cell loss caused by cisplatin treatment. |
| Animal Protocol |
Animal/Disease Models: C57BL/6J wild -type mice at P28 with acute ototoxicity model[2] Doses: 10 mg/kg Route of Administration: intraperitoneal (ip)injection; 10 mg/kg once Experimental Results: Dramatically decreased neomycin- and cisplatin-induced HC loss and demonstrated no effect without neomycin injection with mice. |
| References |
[1]. Aryl Pyrazoles as Potent Inhibitors of Arginine Methyltransferases: Identification of the First PRMT6 ToolCompound. ACS Med Chem Lett. 2015 Apr 6;6(6):655-659. [2]. Inhibition of Protein arginine methyltransferase 6 reduces reactive oxygen species production and attenuates aminoglycoside- and cisplatin-induced hair cell death. Theranostics. 2020 Jan 1;10(1):133-150. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.65 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.65 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.65 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9398 mL | 9.6989 mL | 19.3979 mL | |
| 5 mM | 0.3880 mL | 1.9398 mL | 3.8796 mL | |
| 10 mM | 0.1940 mL | 0.9699 mL | 1.9398 mL |