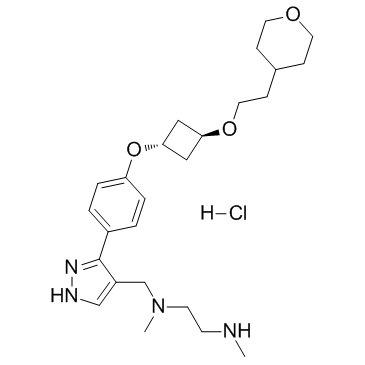
EPZ020411 (EPZ-020411) HCl, the hydrochloride salt of EPZ020411, is a novel and selective inhibitor of PRMT6 (protein arginine methyltransferase, IC50 =10 nM) with therapeutic utility in the treatment of cancer. EPZ020411 shows good bioavailability following subcutaneous dosing in rats making it a suitable tool for in vivo studies.
Physicochemical Properties
| Molecular Formula | C25H39CLN4O3 |
| Molecular Weight | 479.055165529251 |
| Exact Mass | 478.271 |
| CAS # | 2070015-25-5 |
| Related CAS # | EPZ020411;1700663-41-7 |
| PubChem CID | 119081410 |
| Appearance | Off-white to light yellow solid powder |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 33 |
| Complexity | 519 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | Cl.O(CCC1CCOCC1)C1CC(C1)OC1C=CC(C2=C(C=NN2)CN(C)CCNC)=CC=1 |
| InChi Key | LIDZHJMYAKPGOJ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C25H38N4O3.ClH/c1-26-10-11-29(2)18-21-17-27-28-25(21)20-3-5-22(6-4-20)32-24-15-23(16-24)31-14-9-19-7-12-30-13-8-19;/h3-6,17,19,23-24,26H,7-16,18H2,1-2H3,(H,27,28);1H |
| Chemical Name | N,N'-dimethyl-N'-[[5-[4-[3-[2-(oxan-4-yl)ethoxy]cyclobutyl]oxyphenyl]-1H-pyrazol-4-yl]methyl]ethane-1,2-diamine;hydrochloride |
| Synonyms | EPZ020411 EPZ020411 HCl EPZ-020411 EPZ 020411EPZ020411 hydrochloride |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In A375 cells, EPZ020411 hydrochloride (0–20 μM; 24 h) reduces H3R2 methylation[1]. Hair cell survival is increased and neomycin- and cisplatin-induced cell apoptosis is reduced by EPZ020411 hydrochloride (20–40 μM; 6 h)[2]. |
| ln Vivo | In C57BL/6J wild-type mice with acute ototoxicity model, EPZ020411 hydrochloride (10 mg/kg; ip once) decreases neomycin- and cisplatin-induced hearing loss[2]. 1.19 Rats' pharmacokinetic parameters for hydrochloride EPZ020411[1]. Rats IV 1 mg/kg Rats SC 5 mg/kg CL (mL/min/kg) 19.7±1.0 Vss (L/kg) 11.1±1.6 t1/2 (h) 8.54±1.43 9.19±1.60 tmax (h) 0.444 Cmax (ng /mL) 844±306 AUC0-τ (h·ng/mL) 745±34 2456±135 AUC0-inf (h·ng/mL) 846±45 2775±181 F (%) 65.6±4.3 |
| Cell Assay |
Western Blot Analysis[1] Cell Types: A375 cells Tested Concentrations: 0-20 μM Incubation Duration: 24 hrs (hours) Experimental Results: Dose-dependently diminished H3R2 methylation in A375 cells with an IC50 of 0.634 μM. Cell Viability Assay[2] Cell Types: Cultured cochleae cells Tested Concentrations: 20 and 40 μM Incubation Duration: 6 hrs (hours) Experimental Results: Suppressed the apoptotic cascade induced by aminoglycosides and also inhibited cisplatin-induced apoptosis in the hair cells of the cochlear explants after pretreatment deposed. decreased hair cell loss caused by cisplatin treatment. |
| Animal Protocol |
Animal/Disease Models: C57BL/ 6J wild-type mice at P28 with acute ototoxicity model[2] Doses: 10 mg/kg Route of Administration: intraperitoneal (ip)injection; 10 mg/kg once Experimental Results: Dramatically decreased neomycin- and cisplatin-induced HC loss and demonstrated no effect without neomycin injection with mice. |
| References |
[1]. Aryl Pyrazoles as Potent Inhibitors of Arginine Methyltransferases: Identification of the First PRMT6 ToolCompound. ACS Med Chem Lett. 2015 Apr 6;6(6):655-659. [2]. Inhibition of Protein arginine methyltransferase 6 reduces reactive oxygen species production and attenuates aminoglycoside- and cisplatin-induced hair cell death. Theranostics. 2020 Jan 1;10(1):133-150. |
Solubility Data
| Solubility (In Vitro) |
0.1 M HCL : 50 mg/mL (~104.37 mM) DMSO : ~50 mg/mL (~104.37 mM) H2O : ~20 mg/mL (~41.75 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.22 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.22 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (5.22 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 25 mg/mL (52.19 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0874 mL | 10.4371 mL | 20.8742 mL | |
| 5 mM | 0.4175 mL | 2.0874 mL | 4.1748 mL | |
| 10 mM | 0.2087 mL | 1.0437 mL | 2.0874 mL |