EPZ011989 (EPZ-011989) is a potent, orally bioavailable EZH2 inhibitor with high anticancer activity. Inhibitors of the protein methyltransferase Enhancer of Zeste Homolog 2 (EZH2) may have significant therapeutic potential for the treatment of B cell lymphomas and other cancer indications. EPZ011989 displays significant tumor growth inhibition in a mouse xenograft model of human B cell lymphoma. EPZ011989 represents a powerful tool for the expanded exploration of EZH2 activity in biology. Inhibitors of the protein methyltransferase Enhancer of Zeste Homolog 2 (EZH2) may have significant therapeutic potential for the treatment of B cell lymphomas and other cancer indications. The ability of the scientific community to explore fully the spectrum of EZH2-associated pathobiology has been hampered by the lack of in vivo-active tool compounds for this enzyme.
Physicochemical Properties
| Molecular Formula | C35H51N5O4 |
| Molecular Weight | 605.82 |
| Exact Mass | 605.394 |
| Elemental Analysis | C, 69.39; H, 8.49; N, 11.56; O, 10.56 |
| CAS # | 1598383-40-4 |
| Related CAS # | EPZ011989 hydrochloride;2095432-26-9;EPZ011989 trifluoroacetate;1598383-41-5 |
| PubChem CID | 73670548 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 756.7±60.0 °C at 760 mmHg |
| Flash Point | 411.4±32.9 °C |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.596 |
| LogP | 1.34 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 44 |
| Complexity | 1110 |
| Defined Atom Stereocenter Count | 0 |
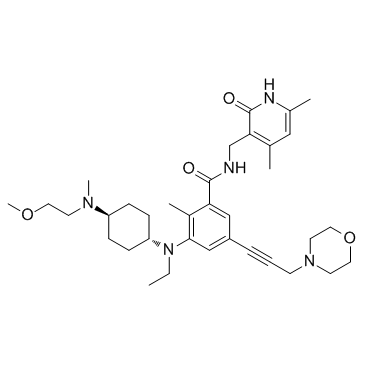
| SMILES | O=C(NCC1=C(C)C=C(C)NC1=O)C2=CC(C#CCN3CCOCC3)=CC(N(CC)[C@H]4CC[C@H](N(CCOC)C)CC4)=C2C |
| InChi Key | XQFINGFCBFHOPE-WXUXXXNLSA-N |
| InChi Code | InChI=1S/C35H51N5O4/c1-7-40(30-12-10-29(11-13-30)38(5)15-18-43-6)33-23-28(9-8-14-39-16-19-44-20-17-39)22-31(27(33)4)34(41)36-24-32-25(2)21-26(3)37-35(32)42/h21-23,29-30H,7,10-20,24H2,1-6H3,(H,36,41)(H,37,42)/t29-,30- |
| Chemical Name | N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-(ethyl((1r,4r)-4-((2-methoxyethyl)(methyl)amino)cyclohexyl)amino)-2-methyl-5-(3-morpholinoprop-1-yn-1-yl)benzamide |
| Synonyms | EPZ-011989 EPZ011989 EPZ 011989. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | EPZ011989 inhibits both wild-type and mutant EZH2 with a Ki value of less than 3 nM[1]. At an IC50 of 94 nM, EPZ011989 lowers cellular H3K27 methylation[1]. EPZ011989 in WSU-DLCL2 (0-10 μM; 11 days) |
| ln Vivo | Oral EPZ011989 (30–1000 mg/kg, single or bid; 7–21 days) has potent anti-tumor action and methyl labeling inhibition [1]. |
| Cell Assay |
cell proliferation assay [1] Cell Types: WSU-DLCL2 Cell Tested Concentrations: 0-10 μM Incubation Duration: 11 days Experimental Results: demonstrated the lowest average in WSU-DLCL2 cells Cytotoxic concentration (LCC). 208 nm. |
| Animal Protocol |
Animal/Disease Models: SCID (severe combined immunodeficient) mouse[1] Doses: 125, 250, 500 and 1000 mg/kg Route of Administration: Oral; single dose twice (two times) daily (BID) for 7 days or twice (two times) daily (BID) for 21 days Experimental Results: LCC coverage was provided for 24 hrs (hrs (hours)) (1000 mg/kg), while the 250 and 500 mg/kg doses provided 24 hrs (hrs (hours)) (1000 mg/kg) coverage for approximately 8 hrs (hrs (hours)). Complete ablation of the methyl label was observed at the end of day 7. Demonstrated potent tumor growth inhibition, diminished methyl labelling, and prolonged total and free plasma exposure. Animal/Disease Models: Rat[1] Doses: 30, 100 and 300 mg/kg Route of Administration: po (po (oral gavage)) Single Experimental Results:Dose (mg/kg) Route t1/2 (h) tmax (h) Cmax (ng/mL ) AUCinf (h*ng/mL) Time above LCC (h) 30 po 4.7 2 240 970 4 100 po 3.9 2.7 1600 5600 8 300 po 3.7 2.7 2900 10000 10 |
| References |
[1]. EPZ011989, A Potent, Orally-Available EZH2 Inhibitor with Robust in Vivo Activity. ACS Med Chem Lett. 2015 Mar 4;6(5):491-495. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~165.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.13 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.13 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.13 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6507 mL | 8.2533 mL | 16.5066 mL | |
| 5 mM | 0.3301 mL | 1.6507 mL | 3.3013 mL | |
| 10 mM | 0.1651 mL | 0.8253 mL | 1.6507 mL |