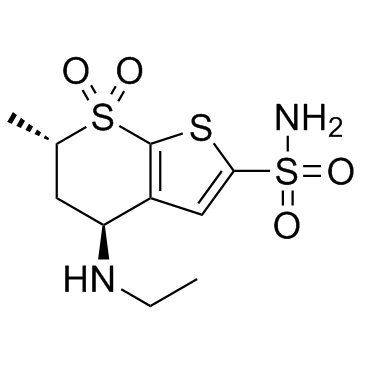
Dorzolamide (L671152; MK507; L671152; MK507; Trusopt) is a CAI (carbonic anhydrase inhibitors) approved for use as an anti-glaucoma agent. It is a water-soluble and potent inhibitor of human carbonic anhydrase II and IV with Ki of 1.9 nM and 31 nM, respectively.
Physicochemical Properties
| Molecular Formula | C10H16N2O4S3 |
| Molecular Weight | 324.44 |
| Exact Mass | 324.027 |
| CAS # | 120279-96-1 |
| Related CAS # | Dorzolamide hydrochloride;130693-82-2;Dorzolamide-d5;1227097-70-2 |
| PubChem CID | 5284549 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.53 g/cm3 |
| Boiling Point | 575.8ºC at 760 mmHg |
| Flash Point | 302ºC |
| Index of Refraction | 1.626 |
| LogP | 4.666 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 19 |
| Complexity | 534 |
| Defined Atom Stereocenter Count | 2 |
| SMILES | O=S(C(S1)=CC2=C1S([C@@H](C)C[C@@H]2NCC)(=O)=O)(N)=O |
| InChi Key | IAVUPMFITXYVAF-XPUUQOCRSA-N |
| InChi Code | InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 |
| Chemical Name | (4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide |
| Synonyms | L671152MK507 L671152 MK507 Trusopt UNII-9JDX055TW1 UNII9JDX055TW1 UNII 9JDX055TW1 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Dorzolamide blocks Component A, which is generated by CO2 influx and hydration by CA-II, in a dose-dependent manner with an IC50 of 2.4 μM (95% confidence interval: 0.5-10.85 μM) for 50% inhibitory concentration [2]. |
| ln Vivo | In an EAC solid tumor model, dorzolamide (3, 10, or 30 mg/kg/day, intraperitoneally) has anticancer efficacy when combined with mitomycin C. The computed ratios (relative values 57.3±1, 25.5±1.8, and 24.3±0.7%, respectively) decline with dorzolamide dosage [3]. |
| Animal Protocol |
Animal/Disease Models: Female Swiss albino mouse (EAC solid tumor) [3]. Doses: 3, 10 or 30 mg/kg/day (with mitomycin C). Doses: IP, one time/day for 3 weeks. Experimental Results: TXNIP and p53 were up-regulated, and bcl-2 was down-regulated. Effectively delays the growth of EAC in mice. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Dorzolamide readily penetrated into the eye in animal studies. Upon ophthalmic administration, dorzolamide is absorbed via the cornea and stroma. Dorzolamide is reported to be absorbed systematically following topical administration. The systemic exposure of dorzolamide following long-term administration was assessed in healthy subjects receiving an oral dose of 2 mg dorzolamide twice daily, which equates to the ophthalmic dose of 2% dorzolamide three times daily. In these subjects receiving the treatment for 20 weeks, the steady-state was reached within 8 weeks. Dorzolamide is primarily excreted unchanged in the urine; however, N-desethyldorzolamide is also detected in the urine. There is limited information on the volume of distribution of dorzolamide; however, the plasma concentrations of dorzolamide and its main metabolite are generally below the assay limit of quantitation, which is 15nM. Dorzolamide accumulates in red blood cells following chronic administration as a result of binding to CA-II, which is contained in peripheral red blood cells (RBCs). There is limited information on the clearance rate of dorzolamide. Metabolism / Metabolites Dorzolamide is slowly metabolised to N-desethyldorzolamide, which has a less potent pharmacological activity on CA-II and some inhibitory effect on CA-I. Like the parent drug, N-desethyldorzolamide is also stored in RBCs, where it binds to CA-I. The findings of an _in vitro_ study using liver microsomes from Sprague-Dawley rats suggest the involvement of CYP2B1, CYP2E1, and CYP3A2 in the metabolism of dorzolamide in rat liver. Biological Half-Life As the drug administration is stopped, dorzolamide stored in RBCs is washed out of RBCs in a non-linear fashion, with the terminal elimination half-life of ≥120 days in RBCs. This initial rapid decline in drug concentrations is followed by the slow elimination phase, where the elimination half-life of the drug is about >4 months. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited experience with the use of ophthalmic dorzolamide indicate that it is unlikely to adversely affect the breastfed infant. French guidelines recommend ophthalmic carbonic anhydrase inhibitor drops as a preferred therapy for glaucoma during breastfeeding. To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants A newborn infant was breastfed during maternal therapy with various combinations of ocular timolol, dipivifrin, dorzolamide, brimonidine and several doses of acetazolamide. Ultimately, the mother was treated with timolol gel-forming solution 0.5% and dorzolamide 2% drops. The drugs were given immediately following breastfeeding with punctal occlusion and no apnea or bradycardia was observed in the infant. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Dorzolamide is approximately 33% bound to plasma proteins. |
| References |
[1]. Whole-blood pharmacokinetics and metabolic effects of the topical carbonic anhydrase inhibitor dorzolamide. Eur J Clin Pharmacol. 1995;47(5):455-60. [2]. Inhibition of carbonic anhydrase activity in cultured bovine corneal endothelial cells by dorzolamide. Invest Ophthalmol Vis Sci. 2002 Oct;43(10):3273-8. [3]. Dorzolamide synergizes the antitumor activity of mitomycin C against Ehrlich's carcinoma grown in mice: role of thioredoxin-interacting protein. Naunyn Schmiedebergs Arch Pharmacol. 2015 Dec;388(12):1271-82. |
| Additional Infomation |
Dorzolamide is 5,6-Dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide in which hydrogens at the 4 and 6 positions are substituted by ethylamino and methyl groups, respectively (4S, trans-configuration). A carbonic anhydrase inhibitor, it is used as the hydrochloride in ophthalmic solutions to lower increased intraocular pressure in the treatment of open-angle glaucoma and ocular hypertension. It has a role as an EC 4.2.1.1 (carbonic anhydrase) inhibitor, an antihypertensive agent and an antiglaucoma drug. It is a sulfonamide and a member of thiophenes. Dorzolamide is a non-bacteriostatic sulfonamide derivative and topical carbonic anhydrase (CA) inhibitor that treats elevated intraocular pressure (IOP) associated with open-angle glaucoma and ocular hypertension. It works by blocking an enzyme in the ciliary process that regulates ion balance and fluid pressure in the eyes. Unlike oral CA inhibitors, dorzolamide has negligible effects of acid-base or electrolyte disturbances and other systemic adverse effects. First marketed in 1995, dorzolamide is available in ophthalmic solutions as monotherapy marketed as Trusopt or in combination with [timolol] as Cosopt PF. Dorzolamide is a Carbonic Anhydrase Inhibitor. The mechanism of action of dorzolamide is as a Carbonic Anhydrase Inhibitor. Dorzolamide is an inhibitor of carbonic anhydrase, a zinc-containing enzyme that catalyzes the rapid conversion of carbon dioxide and water into carbonic acid, protons and bicarbonate ions. Distributed throughout many cells and tissues, various carbonic anhydrases play important roles in mineral and metabolic homeostasis. (NCI04) See also: Dorzolamide Hydrochloride (has salt form). Drug Indication Dorzolamide is indicated for the management of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. It can also be used in combination with [timolol] for the same indication in patients who are insufficiently responsive to ophthalmic beta-blockers. Its pre-operative use was also investigated to prevent elevated intraocular pressure after neodynium yttrium aluminum garnet laser posterior capsulotomy. FDA Label Mechanism of Action Elevated intraocular pressure is a characteristic manifestation of ocular hypertension or open-angle glaucoma. The level of intraocular pressure (IOP) is governed by the balance between the production of aqueous humour (by ocular ciliary processes) and its outflow from the anterior segment of the eye via trabecular (conventional) or uveoscleral (unconventional) pathways. When there is an increase in the resistance to the trabecular outflow of aqueous humour, the intraocular pressure is elevated. Subsequently, optic nerve damage can occur from blood flow restrictions and mechanical distortion of ocular structures. Optic nerve damage can further result in optic disc cupping and progressive visual field loss (and blindness in some cases). Carbonic anhydrase (CA) is a ubiquitous enzyme that catalyzes the reversible hydration of carbon dioxide to bicarbonate ions and dehydration of carbonic acid. In the ocular ciliary processes, the local production of bicarbonate by CAs promotes sodium and fluid transport. CA-II is a key isoenzyme found primarily in red blood cells (RBCs) that regulates aqueous humour production. Dorzolamide is a highly specific CA-II inhibitor, where it displays a 4000-fold higher affinity for carbonic anhydrase II than carbonic anhydrase I. The inhibition of CA-II in the ciliary process disrupts the formation of bicarbonate ions and reduces sodium and fluid transport, which leads to decreased aqueous humour secretion and reduced intraocular pressure. Pharmacodynamics Dorzolamide is a carbonic anhydrase inhibitor that reduces elevated intraocular pressure in open-angle glaucoma or ocular hypertension. When used in combination with topic beta-adrenergic antagonists, dorzolamide has an additive effect of lowering intraocular pressure. The peak ocular hypotensive effect of dorzolamide is observed at about 2 hours following ophthalmic administration. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0822 mL | 15.4112 mL | 30.8223 mL | |
| 5 mM | 0.6164 mL | 3.0822 mL | 6.1645 mL | |
| 10 mM | 0.3082 mL | 1.5411 mL | 3.0822 mL |