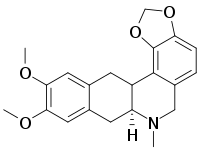
Dicentrine [(S)-form;(+)-Dicentrine] is a naturally occurring an aporphinic alkaloid actin as an α1-adrenoceptor antagonist and is found in several plant species, mainly from family Lauraceae, including Lindera megaphylla. Dicentrine exhibits antinociceptive action in a mouse model of pain at high doses. Potential mode of action: TRPA1-dependent mechanism
Physicochemical Properties
| Molecular Formula | C20H21NO4 |
| Molecular Weight | 339.38504 |
| Exact Mass | 339.147 |
| Elemental Analysis | C, 70.78; H, 6.24; N, 4.13; O, 18.86 |
| CAS # | 517-66-8 |
| PubChem CID | 101300 |
| Appearance | Off-white to light brown solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 480.7±45.0 °C at 760 mmHg |
| Melting Point | 177-178ºC |
| Flash Point | 142.7±25.9 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.616 |
| LogP | 3.98 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 25 |
| Complexity | 502 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | CN1CCC2=CC3=C(C4=C2[C@@H]1CC5=CC(=C(C=C54)OC)OC)OCO3 |
| InChi Key | YJWBWQWUHVXPNC-AWEZNQCLSA-N |
| InChi Code | InChI=1S/C20H21NO4/c1-21-5-4-11-7-17-20(25-10-24-17)19-13-9-16(23-3)15(22-2)8-12(13)6-14(21)18(11)19/h7-9,14H,4-6,10H2,1-3H3/t14-/m0/s1 |
| Chemical Name | (12S)-16,17-dimethoxy-11-methyl-3,5-dioxa-11-azapentacyclo[10.7.1.02,6.08,20.014,19]icosa-1(20),2(6),7,14,16,18-hexaene |
| Synonyms | d-Dicentrine; Dicentrine; Eximine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | α adrenergic receptor |
| Toxicity/Toxicokinetics |
Toxicity Summary Dicentrine was found to be a potent alpha 1-adrenoceptor blocking agent in rat thoracic aorta as revealed by its competitive antagonism of noradrenaline- (pA2 = 8.19 +/- 0.09) or phenylephrine (pA2 = 9.01 +/- 0.10)-induced vasoconstriction. These effects still persisted in denuded aorta. Dicentrine inhibited platelet aggregation and release reaction through the suppression of thromboxane formation and elevation of adenosine 3': S'-cyclic monophosphate. (A15323) d-Dicentrine significantly inhibits the growth of human hepatoma cell line HuH-7 by delaying its doubling time in tissue culture. An in vitro colony forming assay showed that d-dicentrine decreased the colony formation efficiency in both hepatoma cell lines, HuH-7 and MS-G2. An MTT assay in 21 tumor cell lines also revealed that d-dicentrine was most cytotoxic to esophageal carcinoma HCE-6, lymphoma cell lines Molt-4 and CESS, leukemia cell lines HL60 and K562, and hepatoma cell line MS-G2. (A15341) Dicentrine is also a selective α1-adrenoceptor antagonist with potent antiarrhythmic and antihypertensive activities. (A15324) |
| References |
[1]. Effects of dicentrine, a novel α1-adrenoceptor antagonist, on human hyperplastic prostates. European Journal of Pharmacology. 1994 Jan; 252(1):29-34. |
| Additional Infomation |
Dicentrine is an aporphine alkaloid. Dicentrine has been reported in Illigera luzonensis, Lindera megaphylla, and other organisms with data available. Dicentrine is an anticancer compound isolated from Lindera, a species of flowering plants. |
Solubility Data
| Solubility (In Vitro) | DMSO: ~10 mg/mL (~29.5 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (2.95 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (2.95 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 1 mg/mL (2.95 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9465 mL | 14.7323 mL | 29.4646 mL | |
| 5 mM | 0.5893 mL | 2.9465 mL | 5.8929 mL | |
| 10 mM | 0.2946 mL | 1.4732 mL | 2.9465 mL |