Physicochemical Properties
| Molecular Formula | C20H32O6 |
| Molecular Weight | 368.46 |
| CAS # | 64657-20-1 |
| Appearance | Typically exists as solids at room temperature |
| LogP | 0.949 |
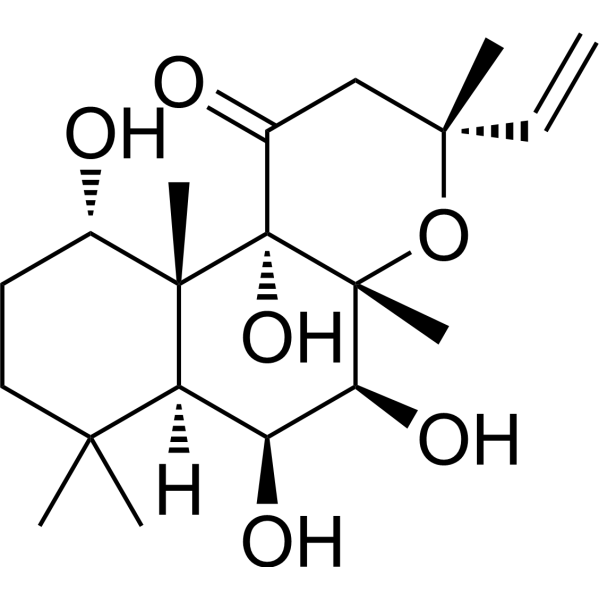
| SMILES | CC1(CCC(C2(C1C(C(C3(C2(C(=O)CC(O3)(C)C=C)O)C)O)O)C)O)C |
| Synonyms | 7-Deacetylforskolin; 7-Desacetylforskolin; 64657-20-1; 1H-Naphtho(2,1-b)pyran-1-one, 3-ethenyldodecahydro-5,6,10,10b-tetrahydroxy-3,4a,7,7,10a-pentamethyl-, (3R-(3alpha,4abeta,5beta,6beta,6aalpha,10alpha,10abeta,10balpha))-; (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-5,6,10,10b-tetrahydroxy-3,4a,7,7,10a-pentamethyl-5,6,6a,8,9,10-hexahydro-2H-benzo(f)chromen-1-one; (3R,4aR,5S,6S,6aS,10S,10aR,10bS)-3-ethenyl-5,6,10,10b-tetrahydroxy-3,4a,7,7,10a-pentamethyl-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-1-one; DTXSID90983272; NS00126397; 3-Ethenyl-5,6,10,10b-tetrahydroxy-3,4a,7,7,10a-pentamethyldodecahydro-1H-naphtho[2,1-b]pyran-1-one |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural product from C. forskohlii. |
| ln Vitro | Forskolin and four analogues of forskolin, 7-beta-[gamma-(N'-methylpiperazino)-butyryloxy]-7-desacet ylforskolin, 7-desacetylforskolin, 7-tosyl-7-desacetylforskolin, and 1,9-dideoxyforskolin, were tested for their ability to activate adenylate cyclase, inhibit glucose transport, and inhibit cytochalasin B binding in rat adipocyte membranes. Forskolin was the most potent analogue in activating adenylate cyclase with an EC50 of 2 microM, whereas 7-beta-[gamma-(N'-methylpiperazino)butyryloxy]-7-desacety lforskolin and 7-desacetylforskolin were less potent, with EC50 values of 3 microM and 20 microM, respectively. The 7-tosyl-7-desacetylforskolin and 1,9-dideoxyforskolin did not stimulate adenylate cyclase even at the highest concentrations tested (100 microM). In contrast, forskolin and all of the analogues were able to fully inhibit glucose transport in adipocyte plasma membranes. The order of potency for the inhibition was forskolin greater than 7-beta-[gamma-(N'-methylpiperazino)butyryloxy]-7-desacety lforskolin greater than 7-desacetylforskolin greater than 7-tosyl-7-desacetylforskolin greater than 1,9-dideoxyforskolin, and the EC50 values were 0.24 microM, 1.8 microM, 7.1 microM, 8.8 microM, and 12.8 microM, respectively. Cytochalasin B binding to rat adipocyte membranes was inhibited by forskolin and the four analogues with the same order of potency as observed for the inhibition of glucose transport. Thus, the site of action of forskolin which is responsible for the inhibition of glucose transport and cytochasin B binding exhibits structural requirements for forskolin and its analogues that are different from those of the site responsible for the activation of adenylate cyclase. [1] |
| References |
[1]. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988 Apr;33(4):449-53. [2]. The antihypertensive and positive inotropic diterpene forskolin: effects of structural modifications on its activity. J Med Chem. 1983 Apr;26(4):486-92. |
| Additional Infomation | Four naturally occurring analogues of forskolin were isolated. Forty-nine semisynthetic derivatives were prepared, incorporating structural alterations at the 1-, 6-, 7-, 9-, 11-, and 14/15-positions. Blood pressure lowering properties of 53 compounds were assessed in anesthetized normotensive cats and of 31 compounds in conscious spontaneously hypertensive (SH) rats. The positive inotropic properties of 25 compounds were investigated in an isolated guinea pig atrial preparation. Forskolin was unique among the compounds in its hypotensive activity in cats and in its positive inotropic properties. Although several derivatives displayed oral antihypertensive activity in the SH rats, none was significantly more potent than forskolin. The optimal structural requirements for activity are apparent, since they are found in forskolin itself. [2] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7140 mL | 13.5700 mL | 27.1400 mL | |
| 5 mM | 0.5428 mL | 2.7140 mL | 5.4280 mL | |
| 10 mM | 0.2714 mL | 1.3570 mL | 2.7140 mL |