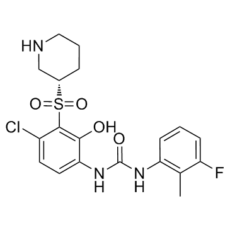
Danirixin (formerly known as GSK-1325756; GSK1325756) is a novel, potent small molecule CXCR2 antagonist being developed as a potential anti-inflammatory drug for COPD. It is a selective, high-affinity and reversible CXCR2 antagonist with IC50 of 12.5 nM for CXCL8. Danirixin, an oral CXCR2 antagonist, has been shown to inhibit agonist-induced neutrophil activation in a dose-dependent manner after single and repeated once-daily oral administration. This suggests that the drug may be useful in treating inflammatory diseases where neutrophils predominate. In many acute and chronic inflammatory diseases, there is an important role for excessive neutrophil activation and presence. One key player in regulating neutrophil extravasation and activation is the CXCR2 chemokine receptor. One possible strategy for decreasing neutrophil migration and activation is selective antagonistic action on the CXCR2 receptor.
Physicochemical Properties
| Molecular Formula | C19H21CLFN3O4S | |
| Molecular Weight | 441.90 | |
| Exact Mass | 441.092 | |
| Elemental Analysis | C, 51.64; H, 4.79; Cl, 8.02; F, 4.30; N, 9.51; O, 14.48; S, 7.25 | |
| CAS # | 954126-98-8 | |
| Related CAS # |
|
|
| PubChem CID | 24780598 | |
| Appearance | White to off-white solid powder | |
| Density | 1.5±0.1 g/cm3 | |
| Boiling Point | 533.1±50.0 °C at 760 mmHg | |
| Flash Point | 276.2±30.1 °C | |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C | |
| Index of Refraction | 1.638 | |
| LogP | 3.93 | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 29 | |
| Complexity | 677 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | S([C@@H]1CNCCC1)(C1C(Cl)=CC=C(NC(=O)NC2C=CC=C(F)C=2C)C=1O)(=O)=O |
|
| InChi Key | NGYNBSHYFOFVLS-LBPRGKRZSA-N | |
| InChi Code | InChI=1S/C19H21ClFN3O4S/c1-11-14(21)5-2-6-15(11)23-19(26)24-16-8-7-13(20)18(17(16)25)29(27,28)12-4-3-9-22-10-12/h2,5-8,12,22,25H,3-4,9-10H2,1H3,(H2,23,24,26)/t12-/m0/s1 | |
| Chemical Name | 1-[4-chloro-2-hydroxy-3-[(3S)-piperidin-3-yl]sulfonylphenyl]-3-(3-fluoro-2-methylphenyl)urea | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCL8-CXCR2 ( IC50 = 12.5 nM ) | ||
| ln Vitro |
|
||
| ln Vivo | Danirixin inhibits CD11b upregulation in rat and human whole-blood experiments measuring neutrophil activation by surface CD11b expression after CXCL2 (rat) or CXCL1 (human) challenge, with pIC50s of 6.05 and 6.3, respectively. With median effective doses (ED50s) of 1.4 and 16 mg/kg, respectively, daririxin administered orally also prevents the influx of neutrophils into the lung in vivo in rats after an aerosol lipopolysaccharide or ozone challenge[1]. | ||
| Enzyme Assay | It is a selective, high-affinity and reversible antagonist of CXCR2 with IC50 of 12.5 nM for CXCL8. | ||
| Animal Protocol |
|
||
| References |
[1]. J Pharmacol Exp Ther . 2017 Aug;362(2):338-346. [2]. BMC Pharmacol Toxicol. 2015, 16: 18. [3]. Drug Metab Dispos . 2010 Mar;38(3):405-14. [4]. Diabetes Obes Metab . 2010 Nov;12(11):1004-12. [5]. Nutrients . 2021 Nov 15;13(11):4088. |
||
| Additional Infomation | Danirixin has been used in trials studying the treatment and basic science of Virus Diseases, Nutritional Status, Pulmonary Disease, Chronic Obstructive, and Infections, Respiratory Syncytial Virus. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2630 mL | 11.3148 mL | 22.6296 mL | |
| 5 mM | 0.4526 mL | 2.2630 mL | 4.5259 mL | |
| 10 mM | 0.2263 mL | 1.1315 mL | 2.2630 mL |