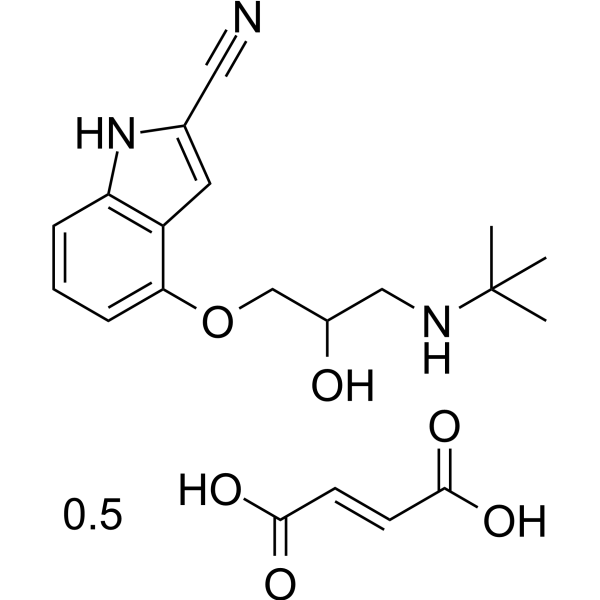
Physicochemical Properties
| Molecular Formula | C16H21N3O2.1/2C4H4O4 |
| Molecular Weight | 345.40 |
| Exact Mass | 690.337 |
| CAS # | 69906-86-1 |
| Related CAS # | 69906-85-0 |
| PubChem CID | 45073413 |
| Appearance | Typically exists as solid at room temperature |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 50 |
| Complexity | 504 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | CC(C)(C)NCC(COC1=CC=CC2=C1C=C(N2)C#N)O.CC(C)(C)NCC(COC1=CC=CC2=C1C=C(N2)C#N)O.C(=C/C(=O)O)\C(=O)O |
| InChi Key | ZSBITJKBOWVCCI-WXXKFALUSA-N |
| InChi Code | InChI=1S/2C16H21N3O2.C4H4O4/c2*1-16(2,3)18-9-12(20)10-21-15-6-4-5-14-13(15)7-11(8-17)19-14;5-3(6)1-2-4(7)8/h2*4-7,12,18-20H,9-10H2,1-3H3;1-2H,(H,5,6)(H,7,8)/b;;2-1+ |
| Chemical Name | (E)-but-2-enedioic acid;4-[3-(tert-butylamino)-2-hydroxypropoxy]-1H-indole-2-carbonitrile |
| Synonyms | CYANOPINDOLOL HEMIFUMARATE; 69906-86-1; 106469-57-2; (E)-but-2-enedioic acid;4-[3-(tert-butylamino)-2-hydroxypropoxy]-1H-indole-2-carbonitrile; Cyanopindolol; 69906-85-0; (+-)-Cyanopindolol; 4-[3-(tert-butylamino)-2-hydroxypropoxy]-1H-indole-2-carbonitrile; 1H-Indole-2-carbonitrile, 4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)-; 4-(3-(tert-butylamino)-2-hydroxypropoxy)-1H-indole-2-carbonitrile; CYANOPINDOLOL(+/-); GTPL132; 4-[3-[tert-Butylamino]-2-hydroxypropoxy]-1H-indole-2-carbonitrilehemifumarate; 4-[3-[tert-Butylamino]-2-hydroxypropoxy]-1H-indole-2-carbonitrile hemifumarate; HMS3267K15; HMS3414I19; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 5-HT receptor |
| ln Vitro | Rat brain cortex slices preincubated with 3H-serotonin were superfused with physiological salt solution containing the serotonin uptake blocker DU 24565 (6-nitroquipazine). The effects of (+/-)-cyanopindolol and its enantiomers, of ICI 118,551 (erythro-dl-1-(7-methylindan-4-yloxy)-3-isopropylaminobut an-2-ol) and of isoprenaline on the electrically (3 Hz) evoked 3H overflow were studied. (+/-)-Cyanopindolol increased the evoked 3H overflow; this effect was prevented by preexposure to the previously characterized serotonin receptor antagonist metitepin. The concentration-response curve of unlabelled serotonin for its inhibitory effect on the electrically evoked 3H overflow was shifted to the right by (+/-)-cyanopindolol (apparent pA2 value: 8.29), whereas that of noradrenaline (determined in the absence of DU 24565) was not affected (apparent pA2 value: less than 6.0). The concentration-response curve of serotonin was also shifted to the right by (-)-cyanopindolol (apparent pA2 value: 8.30) and (+)-cyanopindolol (6.83) but not by ICI 118,551 (less than 5.5). Isoprenaline (up to 10 mumol/l; examined in the absence of DU 24565) did not influence the electrically evoked 3H overflow. The present results show that the presynaptic serotonin autoreceptor is blocked by cyanopindolol in a stereoselective way. This drug is 20 times more potent than metitepin as an antagonist at the presynaptic serotonin autoreceptor, and, in contrast to the latter, it does not act as an antagonist at the presynaptic alpha 2-adrenoceptor on the serotoninergic neurone. [2] |
| ln Vivo | In rat brain cortex slices preincubated with [3H]5-HT, the potencies of 17 5-HT receptor agonists to inhibit the electrically evoked 3H overflow and the affinities of 13 antagonists (including several beta-adrenoceptor blocking agents) to antagonize competitively the inhibitory effect of unlabelled 5-HT on evoked 3H overflow were determined. The affinities of the compounds for 5-HT1B and 5-HT2 binding sites in rat brain cortex membranes (labelled by [125I]cyanopindolol = [125I]-CYP in the presence of 30 mumol/l isoprenaline and [3H]ketanserin, respectively), for 5-HT1A binding sites in pig and rat brain cortex membranes (labelled by [3H]8-hydroxy-2-(di-n-propylamino)tetralin = [3H]8-OH-DPAT) and for 5-HT1C binding sites in pig choroid plexus membranes (labelled by [3H]mesulergine) were also determined. The affinities of the drugs for the various 5-HT recognition sites ranged over 4-5 log units (the functional experiments revealed the same range of differences between the drugs). There were no significant correlations between the affinities of the drugs at 5-HT1C and 5-HT2 binding sites and their potencies or affinities, determined for the 5-HT autoreceptors. In contrast, significant correlations were found between the potencies or affinities of the drugs for the autoreceptors and their affinities at 5-HT1A or 5-HT1B binding sites; the best correlations were obtained with the 5-HT1B binding site. Some of the drugs investigated were not included in the correlation since their agonistic or antagonistic effects on the autoreceptors were weak and pEC30 or apparent pA2 values could not be determined (less than 5.5) [1]. |
| Enzyme Assay | 1. Pindolol, cyanopindolol (CYP) and iodocyanopindolol (IodoCYP) have been reported to act either as antagonists, agonists or partial agonists at the beta 3-adrenoceptor in different preparations. A comprehensive investigation has not yet been described with these compounds tested in one tissue from one species. This study was conducted to delineate the pharmacological effects of pindolol, CYP and IodoCYP and to provide data on their affinities at the predominant beta-adrenoceptor in rat ileum. 2. The beta-adrenoceptors present in rat ileum were characterized in the presence of CGP 20712A and ICI 118 551, atropine and corticosterone, with (-)-isoprenaline used as an agonist. The role of the beta 1 and beta 2-adrenoceptors was determined by the omission of either CGP 20712A, ICI 118 551, or both, from the buffers. Conversely, the effectiveness of the beta 1- and beta 2-adrenoceptor blockade was examined by use of the beta 1-adrenoceptor-selective agonist, RO 363 and the beta 2-adrenoceptor-selective agonist, salbutamol. 3. There was no evidence for the presence of functional beta 1-adrenoceptors, and no strong evidence that beta 2-adrenoceptor stimulation contributed to the relaxant effects of (-)-isoprenaline. (-)-Phenylephrine did not produce relaxation of the tissue and 5-hydroxytryptamine produced contraction. 4. The beta 3-adrenoceptor-selective agonist, BRL 37344 and (-)-isoprenaline were potent full agonists (pD2 8.35 +/- 0.04 and 7.76 +/- 0.14 respectively), whereas ICI D7114 was less potent (pseudo pD2 6.92 +/- 0.15). These results indicate that the predominant functional beta-adrenoceptors in rat ileum are beta 3-adrenoceptors. 5. Partial agonist effects were produced by CYP (pD2 5.28 +/- 0.26) and IodoCYP (pD2 7.0 +/- 0.26), but not pindolol. All three compounds antagonized the effects of (-)-isoprenaline with pKb values of 6.68 +/- 0.10, 7.59 +/- 0.07 and 7.59 +/- 0.11 for pindolol, CYP and IodoCYP respectively. Likewise, CYP and IodoCYP antagonized the effects of BRL 37344 with pKb values of 7.20 +/- 0.22 and 7.21 +/- 0.14 respectively. This study provides the first functional data on the effects of IodoCYP, the ligand with the highest known affinity for the beta 3-adrenoceptor, at the characterized rat ileum beta 3-adrenoceptor. 6. In conclusion, whereas pKb values suggest that CYP and IodoCYP have a similar affinity for the beta 3-adrenoceptor in rat ileum, the higher potency of IodoCYP suggests that it promotes a greater coupling efficiency, or that its partial agonist effects are produced through a site other than the beta 3-adrenoceptor. The similar pKb values for CYP and IodoCYP at the beta 3-adrenoceptor contrast with their order of known affinities at the beta 1- and beta 2-adrenoceptors, where IodoCYP is far more potent than CYP. This provides evidence of further differences in the characteristics of the beta 3-adrenoceptors compared to the beta 1- and beta 2-adrenoceptors. Finally, the utility of IodoCYP as a beta 3-adrenoceptor antagonist would appear to be limited because of the greater magnitude of partial agonist effects that it produces. [1] |
| Animal Protocol | (+/-)[125Iodo] cyanopindolol (ICYP) is a radioligand which binds with an extraordinarily high affinity and specificity to beta-adrenoceptors. In contrast to (+/-) [125Ido]-hydroxybenzylpindolol (IHYP), the new ligand has neither affinity to alpha-nor to 5-HT-receptors. The dissociation constants of ICYP for beta- adrenoceptors in various tissues range from 27 to 40 pM, thereby exceeding the affinity of IHYP by a factor of approximately 3. ICYP does not discriminate between beta 1- and beta 2-adrenoceptors. Therefore, the densities of the two receptor subtypes can be determined from competition curves of ICYP by drugs previously found to show in vitro selectivity for beta 1-adrenoceptors. The guinea pig left ventricle contains only beta 1-adrenoceptors, whereas in a lung tissue, the ratio of beta 1-to beta 2-adrenoceptors is 1 to 4. The calculated affinities of five beta 1-selective antagonists for beta 1-adrenoceptors were nearly identical in the ventricle and the lung. Kinetic studies of ICYP binding to guinea pig lung membranes indicated that the dissociation reaction consists of two components, a fast process (t 1/2 = 9 min) and a slower process (t 1/2 = 8.8 h). A mathematical treatment revealed two possibilities of interpretation: 1. Two forms of the receptor exist which are interconvertible. 2. The (+)- and (-)- enantiomers of ICYP dissociate with different rate constants. The low dissociation constant of ICYP in combination with its high specific radioactivity (2175 Ci mmole -1) allows binding studies to be carried out with small protein and ligand concentrations, e.g. 3 microgram protein per assay in guinea pig lung membranes. Naunyn Schmiedebergs Arch Pharmacol . 1981;317(4):277-85. |
| References |
[1]. Hoey A, et al. Atypical responses of rat ileum to pindolol, cyanopindolol and iodocyanopindolol. Br J Pharmacol. 1996 Feb;117(4):712-6. [2]. Cyanopindolol is a highly potent and selective antagonist at the presynaptic serotonin autoreceptor in the rat brain cortex. Naunyn Schmiedebergs Arch Pharmacol. 1985 Dec;331(4):398-401. [3]. Structural analysis by the comparative molecular field analysis method of the affinity of beta-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors. Eur J Pharmacol. 1993 Jan 4;244(1):77-87. |
| Additional Infomation |
4-[3-(tert-butylamino)-2-hydroxypropoxy]-1H-indole-2-carbonitrile is a member of indoles. Adrenergic beta-Antagonists: Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.) Serotonin Antagonists: Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. |
Solubility Data
| Solubility (In Vitro) | Typically soluble in DMSO (e.g. 10 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8952 mL | 14.4760 mL | 28.9519 mL | |
| 5 mM | 0.5790 mL | 2.8952 mL | 5.7904 mL | |
| 10 mM | 0.2895 mL | 1.4476 mL | 2.8952 mL |