Curcumol is a selective, potent, naturally occuring and pure monomer isolated from Rhizoma Curcumaeis with potential antitumor activities. It shows potent in vitro antiproliferative activity and high in vivo antitumor efficacy.vAs an inducer of apoptosis, curcumol induced cell death in human lung adenocarcinoma (ASTC-a-1) cells by inducing G(2)/M phase arrest, nuclear fragmentation, phosphatidylserine externalization and a rapid translocation of Bax from cytosol into mitochondria. Curcumol also benefits rheumatoid arthritis treatment through suppressing the fibroblast-like synoviocytes (FLS) proliferation and DNA synthesis.
Physicochemical Properties
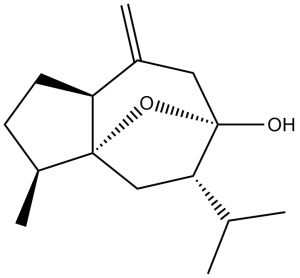
| Molecular Formula | C15H24O2 | |
| Molecular Weight | 236.35 | |
| Exact Mass | 236.177 | |
| CAS # | 4871-97-0 | |
| Related CAS # |
|
|
| PubChem CID | 14240392 | |
| Appearance | White to off-white solid powder | |
| Density | 1.1±0.1 g/cm3 | |
| Boiling Point | 334.5±42.0 °C at 760 mmHg | |
| Melting Point | 141-142ºC | |
| Flash Point | 134.7±22.1 °C | |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C | |
| Index of Refraction | 1.526 | |
| LogP | 3.25 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 1 | |
| Heavy Atom Count | 17 | |
| Complexity | 362 | |
| Defined Atom Stereocenter Count | 5 | |
| SMILES | C[C@H]1CC[C@@H]2[C@]13C[C@H]([C@](O3)(CC2=C)O)C(C)C |
|
| InChi Key | QRMPRVXWPCLVNI-YYFQZIEXSA-N | |
| InChi Code | InChI=1S/C15H24O2/c1-9(2)13-8-14-11(4)5-6-12(14)10(3)7-15(13,16)17-14/h9,11-13,16H,3,5-8H2,1-2,4H3/t11-,12-,13-,14-,15+/m0/s1 | |
| Chemical Name | (3S,3aS,5S,6R,8aS)-Octahydro-3-methyl-8-methylene-5-(1-methylethyl)-6H-3a,6-epoxyazulen-6-ol | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | The principal substance isolated from turmeric root is called curcumin ((-)-Curcumol). By downregulating CDKL3, curcumin inhibits the growth of cholangiocarcinoma cells [2]. Curcumin uses reactive oxygen species and the Akt/GSK3β/cyclin D1 pathway to cause cell cycle arrest in colon cancer cells [3]. Through JNK1/2 and Akt-dependent NF-κB signaling pathways, curcumin inhibits MMP-9 and hence prevents breast cancer cells from metastasizing [4]. | ||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. Curcumol: From Plant Roots to Cancer Roots. Int J Biol Sci. 2019;15(8):1600-1609. Published 2019 Jun 4. [2]. Curcumol Exerts Anticancer Effect in Cholangiocarcinoma Cells via Down-Regulating CDKL3. Front Physiol. 2018;9:234. Published 2018 Mar 20. [3]. Curcumol induces cell cycle arrest in colon cancer cells via reactive oxygen species and Akt/ GSK3β/cyclin D1 pathway. J Ethnopharmacol. 2018;210:1-9. [4]. Curcumol Suppresses Breast Cancer Cell Metastasis by Inhibiting MMP-9 Via JNK1/2 and Akt-Dependent NF-κB Signaling Pathways. Integr Cancer Ther. 2016;15(2):216-225. |
||
| Additional Infomation |
Curcumol is a sesquiterpenoid. Curcumol has been reported in Curcuma aromatica, Curcuma wenyujin, and Cunninghamella blakesleeana with data available. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (10.58 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.2310 mL | 21.1551 mL | 42.3101 mL | |
| 5 mM | 0.8462 mL | 4.2310 mL | 8.4620 mL | |
| 10 mM | 0.4231 mL | 2.1155 mL | 4.2310 mL |