Physicochemical Properties
| Molecular Formula | C10H8N2O2BR2 |
| Molecular Weight | 347.99072 |
| Exact Mass | 345.895 |
| Elemental Analysis | C, 34.51; H, 2.32; Br, 45.92; N, 8.05; O, 9.19 |
| CAS # | 18080-67-6 |
| PubChem CID | 511509 |
| Appearance | White to yellow solid powder |
| LogP | 3.486 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 16 |
| Complexity | 303 |
| Defined Atom Stereocenter Count | 0 |
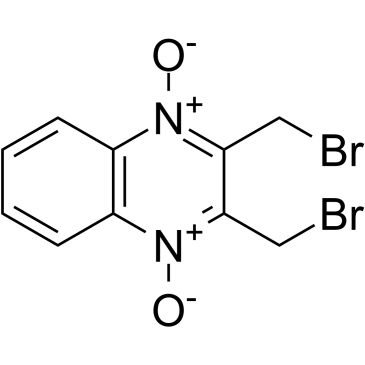
| SMILES | C1=CC=C2C(=C1)N(C(=C(CBr)[N+]2=O)CBr)[O-] |
| InChi Key | DQKNFTLRMZOAMG-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C10H8Br2N2O2/c11-5-9-10(6-12)14(16)8-4-2-1-3-7(8)13(9)15/h1-4H,5-6H2 |
| Chemical Name | 2,3-bis(bromomethyl)-quinoxaline 1,4-dioxide |
| Synonyms | Conoidin A |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Toxoplasma gondii peroxidase II (TgPrxII) |
| ln Vitro | Signal transmission and antioxidant defense are two functions of the widely conserved family of enzymes known as peroxiredoxins. Modifications in PrxII expression have been linked to numerous human illnesses, such as cancer [1]. The enzymatic activity of TgPrxII is inhibited in vitro by conoidin A, which binds to its peroxycysteine. In Ancylostoma ceylonensis, as well as in human PrxII and PrxIV, conoidin A possesses the same level of efficacy when it comes to alkylating or cross-linking the catalytic cysteine. But against mitochondrial hPrxIII, it is ineffectual [2]. The hyperoxidation of mammalian peroxiredoxin I and II by glucose oxidase is inhibited by conoidin A (5 µM) [2]. |
| ln Vivo | The effects of luteolin on ST-segment elevation are blocked by conoidin A (intraperitoneal injection; 5 mg/kg; three days in a row prior to MI/R injury). Furthermore, in the MI/R group, luteolin can lessen the increase in infarct size. Nevertheless, luteolin's effect was neutralized by conoidin A pretreatment. Additionally, luteolin can't lower the activities of CK-MB, AST, and LDH in vivo when cornidin A is pretreated [3]. |
| Enzyme Assay | The knockout vector for TgPrxII was based on the chloramphenicol acetyltransferase selectable marker gene 5′TgPRXII-pTub5CAT-3′TgPRXII, in which the 5′ flanking region (1740 bp) of TgPrxII was amplified by PCR from T. gondii genomic DNA with primers PrxII-1 (5′-CCGGGTACCAGTGGTGTGCGTTCGCG-3′) and PrxII-2 (5′-CCGGGGAACTCGAGTTTCATGC-3′) and cloned between the KpnI and XhoI restriction sites of pTub5CAT in the previously described vector pT/230. The 3′ flanking region (1724 bp) was amplified with primers PrxII-3 (5′-GGAGCGGCCGCCACTCACGGAATGG-3′) and PrxII-4 (5′-CCACCGCGGACCACATAGTGGGCACC-3′) and cloned between the NotI and SacII sites. Stable transformants were generated in RH strain parasites as previously described. TgPrxII knockout parasites were identified by an indirect immunofluorescence assay and confirmed by western blotting using rabbit polyclonal anti-TgPrxII antibodies. Knockout clones KO2 and KO4.1 were isolated by limiting dilution.[1] |
| Cell Assay | Human small airway epithelial cells were incubated for 30 min. with 1, glucose oxidase was added and the cells were incubated for an additional 1.5 hr. Cell extracts were resolved by reducing and non-reducing SDS-PAGE, followed by western blotting with anti-Prx-SO2H/SO3 (Lab Frontier LF-PA0004) as previously described.[1] |
| Animal Protocol |
Animal/Disease Models: Rat myocardial I/R model [3] Doses: 5 mg/kg Route of Administration: intraperitoneal (ip) injection; 5 mg/kg; Three days before MI/R injury Experimental Results: Dramatically reversed the antioxidant effects of luteolin effect. Weaken the protective effect of luteolin. |
| References |
[1]. Jeralyn D Haraldsen, et al. IDENTIFICATION OF CONOIDIN A AS A COVALENT INHIBITOR OF PEROXIREDOXIN II. Org Biomol Chem. 2009;7:3040-3048. [2]. Gu Liu, et al. Optimisation of conoidin A, a peroxiredoxin inhibitor. ChemMedChem. 2010 Jan;5(1):41-5. [3]. Bo Wei, et al. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br J Pharmacol |
Solubility Data
| Solubility (In Vitro) | DMSO : 14.29 ~70 mg/mL ( 41.06 ~201.15 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.43 mg/mL (4.11 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 1.43 mg/mL (4.11 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8736 mL | 14.3682 mL | 28.7365 mL | |
| 5 mM | 0.5747 mL | 2.8736 mL | 5.7473 mL | |
| 10 mM | 0.2874 mL | 1.4368 mL | 2.8736 mL |