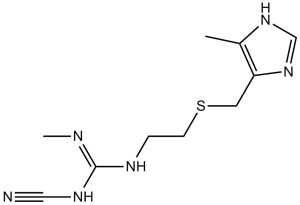
Cimetidine (Tagamet; Cimetag; SKF-92334; Eureceptor; Ulcedine), an approved drug used in the treatment of heartburn and peptic ulcers, is a potent histamine congener that competitively inhibits the binding of histamine to histamine H2 receptors. Cimetidine specifically inhibits the histamine H2-receptor and stops the production of stomach acid.
Physicochemical Properties
| Molecular Formula | C10H16N6S | |
| Molecular Weight | 252.34 | |
| Exact Mass | 252.115 | |
| Elemental Analysis | C, 47.60; H, 6.39; N, 33.30; S, 12.71 | |
| CAS # | 51481-61-9 | |
| Related CAS # | Cimetidine hydrochloride; 70059-30-2; Cimetidine-d3; 1185237-29-9 | |
| PubChem CID | 2756 | |
| Appearance | White crystalline solid | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 476.2±55.0 °C at 760 mmHg | |
| Melting Point | 139-144°C | |
| Flash Point | 241.8±31.5 °C | |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C | |
| Index of Refraction | 1.632 | |
| LogP | 0.07 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 7 | |
| Heavy Atom Count | 17 | |
| Complexity | 296 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | S(C([H])([H])C([H])([H])N([H])/C(=N/C([H])([H])[H])/N([H])C#N)C([H])([H])C1=C(C([H])([H])[H])N([H])C([H])=N1 |
|
| InChi Key | AQIXAKUUQRKLND-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14) | |
| Chemical Name | 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Histamine Receptor ( Ki = 0.6 μM ); H2 Receptor | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Two peak plasma concentrations are often observed after oral administration of cimetidine, likely as a result of discontinuous absorption in the gastrointestinal tract. In healthy patients, the absolute bioavailability of cimetidine is approximately 60%; however, the bioavailability can be as high as 70% in patients with peptic ulcer disease. Overall, rates of bioavailability are much more variable in patients with peptic ulcer disease. Cimetidine is excreted primarily in the urine. The volume of distribution of cimetidine is reported to be 1 L/kg. Cimetidine's reported systemic clearance value is approximately 500-600 ml/min. About 15% of cimetidine is metabolized in the liver. Seventy percent is excreted unchanged in the urine, with fecal losses accounting for approximately 10%. Given orally, cimetidine and ranitidine are almost completely absorbed. Because of first-pass metabolism in the liver, the bioavailability is 50-60%. Both drugs are little bound to plasma proteins (10-20%). Both drugs are mainly excreted in urine - cimetidine up to 90% within 24 hr (50-75% unchanged) and ranitidine up to 60% within 24 hr (about 40% unchanged). The apparent volume of distribution is quite large, in the range of 1.5 l/kg bw, demonstrating that nearly all drug exists outside the intravascular space. Cimetidine is widely distributed throughout the body and is 15-20% bound to plasma proteins. Animal studies indicate that the drug crosses the placenta. Cimetidine is distributed into milk. For more Absorption, Distribution and Excretion (Complete) data for CIMETIDINE (14 total), please visit the HSDB record page. Metabolism / Metabolites After intravenous administration of cimetidine, the majority of the parent drug (58-77%) is eliminated unchanged in the urine. Cimetidine’s primary metabolite is cimetidine sulfoxide and represents an estimated 10-15% of total elimination. Researchers have also identified a minor cimetidine metabolite with a hydroxylated methyl group on the imidazole ring which represents only 4% of total elimination. Both cytochrome P450 enzymes and flavin-containing monooxygenases are implicated in the metabolism of cimetidine, although it is unclear which specific enzymes are involved. Cimetidine is a well known enzyme inhibitor and may impair the metabolism of certain co-administered medications. About 50% to 80% of an intravenous dose is excreted as unchanged drug; 40% of an oral dose is excreted unchanged in the urine in patients with peptic ulcer disease. Most of the remainder of the drug appears in the urine as 5-hydroxymethyl or sulfoxide metabolites. Cimetidine is metabolized in the liver to sulfoxide and 5-hydroxymethyl derivatives, and possibly guanylurea, although this latter compound may result from in vitro degradation. Hepatic Route of Elimination: The principal route of excretion of cimetidine is the urine. Half Life: 2 hours Biological Half-Life Cimetidine's half-life is estimated to be around 2 hours. ELEVEN PATIENTS WITH ASCITIC CIRRHOSIS & ELEVEN PATIENTS WITHOUT LIVER DISEASE RECEIVED 200 MG OF CIMETIDINE ORALLY AND IV. NO DIFFERENCES WERE OBSERVED IN CIMETIDINE T/2 BETWEEN THE 2 GROUPS. CIMETIDINE CLEARANCE WAS DIMINISHED IN CIRRHOTIC PATIENTS (0.426 + OR - 0.138 VERSUS 0.649 + OR - 0.163 L/HR/KG). The elimination half-life in man is 1.9 to 2.2 hours. The plasma elimination half-life is about 2 hours. The half-time for elimination of cimetidine ... is 2 to 3 hours ... . |
||
| Toxicity/Toxicokinetics |
Toxicity Summary Cimetidine binds to an H2-receptor located on the basolateral membrane of the gastric parietal cell, blocking histamine effects. This competitive inhibition results in reduced gastric acid secretion and a reduction in gastric volume and acidity. Hepatotoxicity Chronic therapy with cimetidine has been associated with minor elevations in serum aminotransferase levels in 1% to 4% of patients, but similar rates were reported in placebo recipients. The ALT elevations were usually asymptomatic and transient and usually resolved even without dose modification. Several instances of clinically apparent liver injury have been reported in patients receiving cimetidine, but the time to onset and pattern of injury has varied greatly. Onset can be as short as a few days to as long as 7 months, and the serum enzyme pattern varies from hepatocellular to cholestatic, most cases having a “mixed” hepatocellular-cholestatic pattern of injury (Cases 1 and 2). The injury is rarely severe and resolves within 4 to 12 weeks of stopping cimetidine. Liver biopsy histology often shows prominent centrolobular necrosis. Immunoallergic features (rash, fever, eosinophilia) are uncommon, as is autoantibody formation. Likelihood score: B (highly likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Maternal cimetidine doses of 1000 to 1200 mg daily result in infant dosages that are much less than reported neonatal dosages of 5 to 10 mg/kg daily. Cimetidine would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. However, because of its potential for causing hepatic enzyme inhibition, other drugs might be preferred. Cimetidine can increase serum prolactin and it has been used, but not validated, as a galactogogue. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Histamine H2-receptor blockade is known to stimulate prolactin secretion. In addition, cimetidine may have additional, nonspecific actions that stimulate prolactin secretion. Oral cimetidine doses of 400 mg 4 times daily increased serum prolactin by 50 to 112% in 6 patients. Cimetidine has caused dose-related gynecomastia and galactorrhea in men and nonnursing women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. One clinician reported that she used cimetidine 200 or 300 mg 4 times daily to nursing mothers with a marginal or low milk supply. Subjective reports indicated an increase in milk supply. Protein Binding In humans, approximately 22.5% of cimetidine is plasma protein bound. Interactions OUTPUT OF INTRINSIC FACTOR OF CASTLE IN RESPONSE TO BETAZOLE IS INHIBITED BY CIMETIDINE, BUT BASAL SECRETION OF THE PROTEIN IS ONLY SLIGHTLY AFFECTED AND NO EVIDENCE OF DEFICIENT ABSORPTION OF VITAMIN B12 HAS BEEN NOTED, EVEN DURING LONG-TERM TREATMENT. Antacids reduce the oral bioavailability of concomitantly administered cimetidine or ranitidine. ... These drugs probably should be given one hour apart. REDUCED HEPATIC BLOOD FLOW /AFTER ADMIN OF CIMETIDINE/ HAS BEEN REPORTED TO PROLONG THE CLEARANCE AND, THEREFORE, EXAGGERATE THE EFFECTS OF MORPHINE ... AND LIDOCAINE. SIX OUT OF 8 PATIENTS TREATED WITH CARMUSTINE, 80 MG/SQ M/DAY FOR 3 DAYS, CIMETIDINE, 300 MG 6 HOURLY, AND STEROIDS DEMONSTRATED MARKED LEUCOPENIA AND THROMBOCYTOPENIA ... AFTER FIRST ADMIN. IN COMPARISON ONLY 6 OUT OF 40 PATIENTS WHO WERE SIMILARLY TREATED, BUT WITHOUT CIMETIDINE, SHOWED COMPARABLE WHITE CELL AND PLATELET DEPRESSION. For more Interactions (Complete) data for CIMETIDINE (37 total), please visit the HSDB record page. |
||
| References |
[1]. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6802-7. [2]. Cimetidine induces interleukin-18 production through H2-agonist activity in monocytes. Mol Pharmacol, 2006. 70(2): p. 450-3. [3]. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther, 2013. 94(5): p. 585-92. [4]. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol Immunol, 2013. 54(1): p. 74-83. [5]. Cimetidine inhibits salivary gland tumor cell adhesion to neural cells and induces apoptosis by blocking NCAM expression. BMC Cancer, 2008. 8: p. 376. [6]. Cimetidine Reduces the Alveolar Bone Loss in Induced Periodontitis in Rat Molars. J Periodontol, 2013. |
||
| Additional Infomation |
Therapeutic Uses Adjuvants, Immunologic; Analgesics, Non-Narcotic; Anti-Ulcer Agents; Enzyme Inhibitors; Histamine H2 Antagonists IN MAN, A SINGLE DOSE (300 MG) WILL INHIBIT BASAL (FASTING) SECRETION AND ALSO SECRETION INDUCED BY SOLID, LIQ, OR PEPTONE MEALS, SHAM FEEDING, FUNDIC DISTENTION, PENTAGASTRIN, BETHANECHOL, INSULIN, AND CAFFEINE, AS WELL AS THE PHYSIOLOGICAL STIMULUS PROVIDED BY EATING. ... THIS SPECTRUM INCL THE CEPHALIC OR VAGAL PHASE. CIMETIDINE IS THE PREFERRED ALTERNATIVE FOR MANY PATIENTS WHO CANNOT OR WILL NOT TOLERATE AN INTENSIVE, PROLONGED ANTACID REGIMEN. Cimetidine is a useful alternative to antacids in preventing aspiration pneumonitis during childbirth and elective surgical procedures. It is less useful than antacids during emergency surgery because of its slow onset of action. This drug had been given to prevent alkalosis in patients subjected to prolonged nasogastric aspiration, especially those secreting large amounts of acid, and to decrease ileostomy/jejunostomy output in the short bowel syndrome. For more Therapeutic Uses (Complete) data for CIMETIDINE (21 total), please visit the HSDB record page. Drug Warnings DESPITE POOR PENETRATION TO THE CNS, NEURAL DYSFUNCTION HAS BEEN ENCOUNTERED, PARTICULARLY WITH HIGH DOSES IN ELDERLY PATIENTS AND IN ASSOCIATION WITH IMPAIRED RENAL EXCRETION. THE EFFECTS INCL CONFUSION, SLURRED SPEECH, DELIRIUM, HALLUCINATIONS, AND COMA. IN SOME INSTANCES, WITHDRAWAL OF CIMETIDINE AFTER A PERIOD OF TREATMENT HAS BEEN FOLLOWED BY RELAPSES IN THE SYMPTOMS OF ULCER AND EVEN BY PERFORATION OF DUODENAL, ESOPHAGEAL, OR GASTRIC ULCERS. ... CIMETIDINE IS INEFFECTIVE IN ACUTE OR ALCOHOLIC PANCREATITIS AND IT MAY ACTUALLY INCR AND PROLONG HYPERAMYLASEMIA. ... CLIN EXPERIENCE IN CHILDREN IS EXTREMELY LIMITED, AND THE BENEFIT/RISK RATIO SHOULD BE CONSIDERED CAREFULLY. For more Drug Warnings (Complete) data for CIMETIDINE (15 total), please visit the HSDB record page. Pharmacodynamics Cimetidine is a histamine H2-receptor antagonist. It reduces basal and nocturnal gastric acid secretion and a reduction in gastric volume, acidity, and amount of gastric acid released in response to stimuli including food, caffeine, insulin, betazole, or pentagastrin. It is used to treat gastrointestinal disorders such as gastric or duodenal ulcer, gastroesophageal reflux disease, and pathological hypersecretory conditions. Cimetidine inhibits many of the isoenzymes of the hepatic CYP450 enzyme system. Other actions of Cimetidine include an increase in gastric bacterial flora such as nitrate-reducing organisms. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 3 mg/mL (11.89 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (11.89 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 3 mg/mL (11.89 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 3.12 mg/mL (12.36 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9629 mL | 19.8145 mL | 39.6291 mL | |
| 5 mM | 0.7926 mL | 3.9629 mL | 7.9258 mL | |
| 10 mM | 0.3963 mL | 1.9815 mL | 3.9629 mL |