Description: Camalexin is a naturally occuring indole-based phytoalexin isolated from Camelina sativa and Arabidopsis (Cruciferae) with antibacterial, antifungal, antiproliferative and anticancer activities. Camalexin can induce reactive oxygen species (ROS) production. Camalexin has been used in quantification and tolerance assays in Arabidopsis thaliana leaves.
Physicochemical Properties
| Molecular Formula | C11H8N2S |
| Molecular Weight | 200.25962 |
| Exact Mass | 200.041 |
| CAS # | 135531-86-1 |
| PubChem CID | 636970 |
| Appearance | White to yellow solid powder |
| Melting Point | 134 - 137 °C |
| LogP | 3.291 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 14 |
| Complexity | 210 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | IYODIJVWGPRBGQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C11H8N2S/c1-2-4-10-8(3-1)9(7-13-10)11-12-5-6-14-11/h1-7,13H |
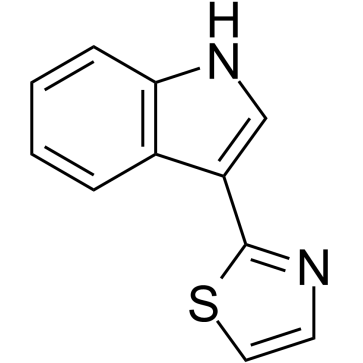
| Chemical Name | 2-(1H-indol-3-yl)-1,3-thiazole |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Human breast cancer cell lines have antiproliferative activity in response to camalexin [2]. In oomycetes, the first steps toward the synthesis of Camalexin and the production of reactive oxygen species (ROS) are Phytophthora and Pythium Nep1-like proteins (necrosis and ethylene-inducing peptide 1-like proteins). ROS seem to have a ubiquitous role in the synthesis of camalexin. Camalexin synthesis and chemical stimulation of ROS (e.g., by applying acifluorfen) happen at the same time. An esa1 mutant with delayed Camalexin induction was found in a screen for enhanced susceptibility to Alternaria brassicae. The amounts of produced Camalexin are decreased, especially in reaction to ROS inducers. The fact that esa1 mutants are unable to produce Camalexin in response to Leptosphaeria maculans confirms the crucial role that ESA1 plays. Ups1, a different mutant that dramatically lowers Camalexin accumulation, was identified due to decreased tryptophan biosynthesis enzyme expression [2]. |
| References |
[1]. Synthesis of camalexin and related phytoalexins. Tetrahedron. Volume 48, Issue 14, 1992, Pages 2919-2924. [2]. Glawischnig E. Camalexin. Phytochemistry. 2007 Feb;68(4):401-6. [3]. Antiproliferative and cancer chemopreventive activity of phytoalexins: focus on indole phytoalexins from crucifers. Neoplasma. 2003;50(4):239-45. |
| Additional Infomation |
Camalexin is an indole phytoalexin that is indole substituted at position 3 by a 1,3-thiazol-2-yl group. It has a role as a metabolite. It is an indole phytoalexin and a member of 1,3-thiazoles. Camalexin has been reported in Arabidopsis, Arabidopsis thaliana, and Camelina sativa with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~125 mg/mL (~624.19 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (10.39 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (10.39 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (10.39 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.9935 mL | 24.9675 mL | 49.9351 mL | |
| 5 mM | 0.9987 mL | 4.9935 mL | 9.9870 mL | |
| 10 mM | 0.4994 mL | 2.4968 mL | 4.9935 mL |