CPI-455 is a novel, potent and specific inhibitor of the KDM5 demethylase which catalyzes the demethylation of histone H3 on lysine 4 (H3K4) and is required for the survival of drug-tolerant persister cancer cells (DTPs). CPI-455 elevates the global levels of H3K4 trimethylation (H3K4me3) and decreases the number of DTPs in multiple cancer cell line models treated with standard chemotherapy or targeted agents. CPI-455 has improved potency against KDM5A while demonstrating ~200-fold selectivity for KDM5A over KDM4C. CPI-455 inhibits KDM5A, KDM5B and KDM5C to similar extents but showed substantially weaker potency toward KDM4C and KDM7B (~200- and 770-fold, respectively) and no measurable inhibition of KDM2B, KDM3B or KDM6A. CPI-455-mediated KDM5 inhibition results in a dose-dependent increase in global H3K4me3 in HeLa cells.
Physicochemical Properties
| Molecular Formula | C16H15CLN4O | |
| Molecular Weight | 314.77 | |
| Exact Mass | 278.116 | |
| Elemental Analysis | C, 69.05; H, 5.07; N, 20.13; O, 5.75 | |
| CAS # | 1628208-23-0 | |
| Related CAS # | CPI-455 hydrochloride;2095432-28-1 | |
| PubChem CID | 78426698 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 488.7±55.0 °C at 760 mmHg | |
| Flash Point | 249.3±31.5 °C | |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C | |
| Index of Refraction | 1.670 | |
| LogP | 2.46 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 21 | |
| Complexity | 611 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | 0 |
|
| InChi Key | VGXRQCOVGLGFIM-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C16H14N4O/c1-10(2)13-14(11-6-4-3-5-7-11)19-15-12(8-17)9-18-20(15)16(13)21/h3-7,9-10,18H,1-2H3 | |
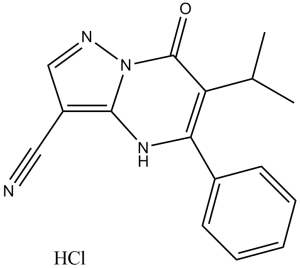
| Chemical Name | 7-oxo-5-phenyl-6-propan-2-yl-1H-pyrazolo[1,5-a]pyrimidine-3-carbonitrile | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | KDM5 |
| ln Vitro | Several cancer cell line models treated with normal chemotherapy or targeted medicines show that CPI-455 reduces the amount of DTPs, raises overall levels of H3K4 trimethylation (H3K4me3), and facilitates KDM5 inhibition [1]. High affinity for the target KDM5 protein is possessed by CPI-455. Within 24 hours of exposure to either of the two active drugs, CPI-455 and CPI-766, a dose-dependent elevation in H3K4me3 was seen. Three luminal breast cancer cell lines, MCF-7, T-47, and EFM-19, had computed IC50 values of 35.4, 26.19, and 16.13 μM for the KDM5 inhibitor CPI0455, respectively [2]. |
| ln Vivo | Mice treated with CPI-455 (50/70 mg/kg, intraperitoneal injection, daily) for dual blockade of B7-H4 and KDM5B developed protective immunity [2]. |
| Enzyme Assay | The KDM5 family of histone demethylases catalyzes the demethylation of histone H3 on lysine 4 (H3K4) and is required for the survival of drug-tolerant persister cancer cells (DTPs). Here we report the discovery and characterization of the specific KDM5 inhibitor CPI-455. The crystal structure of KDM5A revealed the mechanism of inhibition of CPI-455 as well as the topological arrangements of protein domains that influence substrate binding. CPI-455 mediated KDM5 inhibition, elevated global levels of H3K4 trimethylation (H3K4me3) and decreased the number of DTPs in multiple cancer cell line models treated with standard chemotherapy or targeted agents. These findings show that pretreatment of cancer cells with a KDM5-specific inhibitor results in the ablation of a subpopulation of cancer cells that can serve as the founders for therapeutic relapse[1]. |
| Cell Assay |
Chemotaxis assay[3] CD8+ lymphocytes were selected from PBMCs by negative selection using magnetic beads and cultured with anti-CD3/anti-CD28–coated beads for 7 days to generate CD8+ effector cells. These cells were loaded into the upper chambers of transwell inserts (5.0-μm pore size). In the bottom well, medium containing different amounts of neutralization antibodies to chemokines, or culture supernatant from P. gingivalis–infected cell lines (Kyse-410 and Kyse-150), was added. The KDM5B inhibitor CPI-455 was used. For antibody blocking assays, neutralization anti-CXCL9 (MAB392), anti-CXCL10 (MAB266), and anti-CXCL11 were added into culture supernatants and incubated at 37°C for 30 minutes before adding into T cells. The contents of the lower chamber were collected, and the percentage of CD8+ cells was determined by FACS. |
| Animal Protocol |
Animal/Disease Models: Sixweeks old male C57BL/6 mice (One- to 2-mm fragments of P. gingivalis–positive PDXs were implanted subcutaneously (sc) into the flank region of humanized mice.) Doses: 50 mg/kg or 70 mg/ kg (combined with anti–B7-H4). Route of Administration: IP, daily, 14-28 days. Experimental Results: Histopathology analysis revealed no inflammation in either group at 2 weeks in response to the primary infection. However, at 8 weeks after inoculation, mice receiving monotherapy demonstrated mild inflammation, whereas the combined treatment presented with heavy to severe inflammation, which persisted at 12 and 16 weeks after challenge. Treatment with CPI-455 to selectively target H3K4-specific JmjC demethylases increased CXCL11, CXCL9, and CXCL10 following infection , with maximum levels observed 48 hrs (hours) after infection. In vivo tumor studies[3] One- to 2-mm fragments of P. gingivalis–positive PDXs were implanted subcutaneously into the flank region of humanized mice. After injection, the mice were randomly divided into different groups (n = 10/group). Mice were treated with CPI-455 (50 mg/kg or 70 mg/kg, daily, intraperitoneal injection) and anti–B7-H4 (188; 500 μg/mouse, weekly, intraperitoneal injection), followed by the sequential administration of CPI-455 and anti–B7-H4 Ab started on days 6 and 20, respectively; phased combined treatment for 14 days with CPI-455 started on day 6 and combined treatment with anti–B7-H4 Ab started on day 13 and continuing for 3 weeks; or extended phased combined treatment with CPI-455 for 28 days. The animals dosed according to the appropriate schema (n = 10 mice/group) were monitored daily for up to 2 months, and the objective response rate and survival were recorded.[3] An additional cohort of mice (n = 5/group) was included to conduct mechanistic studies. In this cohort, the mice were sacrificed on day 30 after tumor inoculation. Residual tumors were surgically removed before terminal escape (tumor with partial response, PR) or complete remission (tumor with complete response) and processed for IHC and flow cytometry analysis. IHC and flow cytometry results related to lymphocyte infiltration were determined, and a representative mouse from each treatment group [(i) animals receiving control therapy, (ii) animals receiving anti–B7-H4 Ab monotherapy, (iii) animals receiving 75 mg/kg CPI-455 monotherapy, and (iv) animals treated with extended phased therapy using 75 mg/kg dose CPI-455] in this separate cohort is shown, TV (mm3) = π/6 × length × width2. Mice suffering from progressive disease or those used for subsequent analysis were euthanized when the TV was more than 2,500 mm3. |
| References |
[1]. An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells. Nat Chem Biol. 2016 Jul;12(7):531-8. [2]. Benjamin R. Leadem. NOVEL HISTONE DEMETHYLASE INHIBITORS SYNERGISTICALLY. [3]. Blockade of Immune-Checkpoint B7-H4 and Lysine Demethylase 5B in Esophageal Squamous Cell Carcinoma Confers Protective Immunity against P. gingivalis Infection. Cancer Immunol Res. 2019 Sep;7(9):1440-1456. |
| Additional Infomation | Pathogens are capable of hijacking immune defense mechanisms, thereby creating a tolerogenic environment for hypermutated malignant cells that arise within the site of infection. Immune checkpoint-oriented immunotherapies have shown considerable promise. Equally important, the epigenetic reprogramming of an immune-evasive phenotype that activates the immune system in a synergistic manner can improve immunotherapy outcomes. These advances have led to combinations of epigenetic- and immune-based therapeutics. We previously demonstrated that Porphyromonas gingivalis isolated from esophageal squamous cell carcinoma (ESCC) lesions represents a major pathogen associated with this deadly disease. In this study, we examined the mechanisms associated with host immunity during P. gingivalis infection and demonstrated that experimentally infected ESCC responds by increasing the expression of B7-H4 and lysine demethylase 5B, which allowed subsequent in vivo analysis of the immunotherapeutic effects of anti-B7-H4 and histone demethylase inhibitors in models of chronic infection and immunity against xenografted human tumors. Using three different preclinical mouse models receiving combined therapy, we showed that mice mounted strong resistance against P. gingivalis infection and tumor challenge. This may have occurred via generation of a T cell-mediated response in the microenvironment and formation of immune memory. In ESCC subjects, coexpression of B7-H4 and KDM5B correlated more significantly with bacterial load than with the expression of either molecule alone. These results highlight the unique ability of P. gingivalis to evade immunity and define potential targets that can be exploited therapeutically to improve the control of P. gingivalis infection and the development of associated neoplasia.[3] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1769 mL | 15.8846 mL | 31.7692 mL | |
| 5 mM | 0.6354 mL | 3.1769 mL | 6.3538 mL | |
| 10 mM | 0.3177 mL | 1.5885 mL | 3.1769 mL |