|
CPI-169 (CPI169) is a novel, potent, and selective inhibitor of EZH2 (enhancer of zeste homolog 2) inhibitor with anticancer activity. It inhibits EZH2 WT, EZH2 Y641N, and EZH1 with IC50s of 0.24 nM, 0.51 nM, and 6.1 nM, respectively. CPI-169 exhibits excellent antiproliferative activity and high in vivo antitumor efficacy in a NHL xenograft model.
|
Physicochemical Properties
| Molecular Formula |
C27H36N4O5S
|
| Molecular Weight |
528.66
|
| Exact Mass |
528.24
|
| CAS # |
1450655-76-1
|
| Related CAS # |
|
| PubChem CID |
78357814
|
| Appearance |
Typically exists as solid at room temperature
|
| Density |
1.3±0.1 g/cm3
|
| Index of Refraction |
1.631
|
| LogP |
2.67
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
6
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
37
|
| Complexity |
1040
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
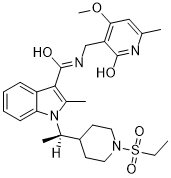
O=C(C1=C(C)N(C(C2CCN(S(=O)(CC)=O)CC2)C)C3=C1C=CC=C3)NCC4=C(OC)C=C(C)NC4=O
|
| InChi Key |
LHGUZCKPFXXVPV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H36N4O5S/c1-6-37(34,35)30-13-11-20(12-14-30)18(3)31-19(4)25(21-9-7-8-10-23(21)31)27(33)28-16-22-24(36-5)15-17(2)29-26(22)32/h7-10,15,18,20H,6,11-14,16H2,1-5H3,(H,28,33)(H,29,32)
|
| Chemical Name |
1-[1-(1-ethylsulfonylpiperidin-4-yl)ethyl]-N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methylindole-3-carboxamide
|
| Synonyms |
| CPI 169; CPI169; CPI-169;(R,Z)-1-(1-(1-(ethylsulfonyl)piperidin-4-yl)ethyl)-N-((2-hydroxy-4-methoxy-6-methylpyridin-3-yl)methyl)-2-methyl-1H-indole-3-carbimidic acid |
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder-20°C 3 years
4°C 2 years
In solvent -80°C 6 months
-20°C 1 month
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
|
Biological Activity
| ln Vitro |
In vitro activity: In KARPAS-422 cells, CPI-169 shows a dose-dependent inhibitory effect on cell viability, and produces synergy anti-proliferative activity when used in combination with ABT-199. In 16 out of 25 NHL cell lines, CPI-169 also suppresses cell growth with GI50 of<5 μM.
Kinase Assay: Compound potency is also assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, PRC2 containing either EZH1 (160 pM), wt EZH2 (40 pM), or Y641N mutant EZH2 (80 pM, both EZH2 prepared in-house) is pre-incubated with 3H-SAM (0.9 µM), 2 µM H3K27me3 activating peptide (H2N-RKQLATKAAR(Kme3)SAPATGGVKKP-amide) and compounds (as 10 point duplicate dose response titrations) for 120 min in a buffer consisting of 50 mM Tris (pH 8.5), 1 mM DTT, 0.07 mM Brij-35, 0.1% BSA, and 0.8% DMSO in a total volume of 12.5 µl in a black 384 well plate. Reaction is initiated with biotinylated H3 substrate peptides (H3K27me1 for wt EZH2, H3K27me2 for Y641N mutant EZH2; H2N-RKQLATKAAR(Kmen)SAPATGGVKKP-NTPEGBiot) as a 2 µM stock in 12.5 µL and allowed to react at room temperature for 5 h. Quenching is accomplished by addition of 20 µl of STOP solution (50 mM Tris (pH 8.5), 200 mM EDTA, 2 mM SAH). 35 µL of the quenched solution is transferred to Streptavidin Flashplates, incubated overnight, washed, and read in a TopCount Reader. For titrations all compound dilutions are in DMSO, final DMSO concentrations are 0.8% (v/v), and turnover is kept to less than < 5%. IC50s are calculated using non-linear least square four parameter fits (GraphPad 6.0).
Cell Assay: Relative cell numbers are assessed by Cell Titer-Glo (CTG) luminescent cell viability assay using an Envision instrument. GraphPad Prism 6.0 is used for curve fitting, IC50/GI50 and Hill coefficient (H) calculations. The GI90 is calculated using the formula: EC90 = (90 /100-90)1/H * EC50. Cell line: 25 NHL cell lines |
|
| ln Vivo |
| In mice bearing KARPAS-422 xenografts, CPI-169 (200 mg/kg, s.c.) effectively suppresses H3K27me3 levels and results in lymphoma tumor regression without affecting body weight or causing any overt adverse effects. |
|
| Animal Protocol |
| Dissolved in 10% DMSO + 60% polytheylene glycol 400 + 30% ddH2O; 200 mg/kg; s.c. injection | | Mice bearing KARPAS-422 subcutaneous xenografts | |
|
| References |
Chem Biol.2014 Nov 20;21(11):1463-75.
|
| Additional Infomation |
1-[1-(1-ethylsulfonyl-4-piperidinyl)ethyl]-N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-3-indolecarboxamide is an indolecarboxamide.
|
|
Solubility Data
| Solubility (In Vitro) |
| DMSO: 100 mg/mL (189.1 mM) | | Water:<1 mg/mL | | Ethanol:100 mg/mL (189.1 mM) |
|
| Solubility (In Vivo) |
| 10% DMSO+60% PEG 400+30% ddH2O: 30mg/mL |
(Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions |
|
1 mg |
5 mg |
10 mg |
| 1 mM |
1.8916 mL |
9.4579 mL |
18.9157 mL |
| 5 mM |
0.3783 mL |
1.8916 mL |
3.7831 mL |
| 10 mM |
0.1892 mL |
0.9458 mL |
1.8916 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles. |