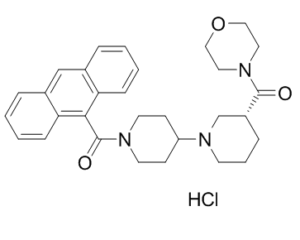
CP-640186 HCl is a novel and potent inhibitor of mammalian ACCs (isozyme-nonselective acetyl-CoA carboxylase) with IC50s of 53 nM and 61 nM for rat liver ACC1 and rat skeletal muscle ACC2 respectively; It hash improved metabolic stability in comparison to CP-610431, an analog of CP-640186. Inhibition of ACC, with its resultant inhibition of fatty acid synthesis and stimulation of fatty acid oxidation, has the potential to favorably affect the multitude of cardiovascular risk factors associated with the metabolic syndrome. CP-640186 can reduce body weight and improve insulin sensitivity in test animals. CP-640186, also inhibited both isozymes with IC50s of ~55 nM but was 2–3 times more potent than CP-610431 in inhibiting HepG2 cell fatty acid and TG synthesis. CP-640186 also stimulated fatty acid oxidation in C2C12 cells (ACC2) and in rat epitrochlearis muscle strips with EC50s of 57 nM and 1.3 uM.
Physicochemical Properties
| Molecular Formula | C30H36CLN3O3 | |
| Molecular Weight | 522.08 | |
| Exact Mass | 521.244 | |
| CAS # | 591778-70-0 | |
| Related CAS # | CP-640186;591778-68-6 | |
| PubChem CID | 23589188 | |
| Appearance | Light yellow to pink solid powder | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 3 | |
| Heavy Atom Count | 37 | |
| Complexity | 753 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | C1C[C@H](CN(C1)C2CCN(CC2)C(=O)C3=C4C=CC=CC4=CC5=CC=CC=C53)C(=O)N6CCOCC6.Cl |
|
| InChi Key | DUBNXJIOBFRASV-GJFSDDNBSA-N | |
| InChi Code | InChI=1S/C30H35N3O3.ClH/c34-29(32-16-18-36-19-17-32)24-8-5-13-33(21-24)25-11-14-31(15-12-25)30(35)28-26-9-3-1-6-22(26)20-23-7-2-4-10-27(23)28;/h1-4,6-7,9-10,20,24-25H,5,8,11-19,21H2;1H/t24-;/m1./s1 | |
| Chemical Name | [(3R)-1-[1-(anthracene-9-carbonyl)piperidin-4-yl]piperidin-3-yl]-morpholin-4-ylmethanone;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Treatment with CP-640186 (20 µM; 48 h) can stop H460 cell growth[3]. In C2C12 cells and muscle strips, CP-640186 (0.1 nM-100 µM; 2 h) treatment boosts fatty acid metabolism in a concentration-dependent manner [1]. In HepG2 cells, CP-640186 (0.62-1.8 µM; 2 h) treatment suppresses the synthesis of TG and fatty acids[1]. |
| ln Vivo | The acute effectiveness of CP-640186 (oral gavage; 4.6–21 mg/kg; once) has been demonstrated[1]. When administered at equal doses, CP-640186 (intravenous injection and oral gavage; intravenous dose: 5 mg/kg; oral dose: 10 mg/kg; once) causes less drug exposure in rats than in ob/ob mice[1]. At high exposure levels, CP-640186 (oral gavage; 100 mg/kg; once) treatment completely switches the body's energy source from using carbohydrates to using fatty acids[1]. |
| Cell Assay |
Cell Proliferation Assay[3] Cell Types: Human fibroblasts and H460 cells Tested Concentrations: 20 µM Incubation Duration: 48 hrs (hours) Experimental Results: Led to a ∼30% decrease in cell number compared to vehicle-treated controls. Cell Viability Assay[1] Cell Types: C2C12 cells and muscle strips Tested Concentrations: 0.1 nM-100 µM Incubation Duration: 2 hrs (hours) Experimental Results: Stimulated palmitate acid oxidation with an EC50 of 57 nM and a maximal stimulation of 280% in C2C12 cells. Stimulated palmitate acid oxidation with an EC50 of 1.3 μM and a maximal stimulation of 240% in isolated rat epitrochlearis muscle. Cell Viability Assay[1] Cell Types: HepG2 cells Tested Concentrations: 0.62-1.8 µM Incubation Duration: 6 hrs (hours) Experimental Results: Inhibited fatty acid synthesis and TG synthesis in HepG2 cells with EC50s of 0.62 μM and 1.8 μM, respectfully. |
| Animal Protocol |
Animal/Disease Models: Male ob/ob mice[1] Doses: 4.6-21 mg/kg Route of Administration: po (oral gavage); 4.6-21 mg/kg; once Experimental Results: Demonstrated acute efficacy for up to 8 h after oral administration, exhibiting ED50 values of 4.6, 9.7, and 21 mg/kg, at 1, 4, and 8 h, respectively, after treatment. Animal/Disease Models: Male SD (Sprague-Dawley) rats[1] Doses: intravenous (iv) dose, 5 mg/kg; oral dose, 10 mg/kg Route of Administration: intravenous (iv) injection and po (oral gavage); intravenous (iv) dose, 5 mg/kg; oral dose, 10 mg/kg; once Experimental Results: demonstrated a plasma half-life of 1.5 h, a bioavailability of 39%, a Clp of 65 ml/min/kg, a Vdss of 5 liters/kg, an oral Tmax of 1.0 h, an oral Cmax of 345 ng/mL, and an oral AUC0-∞ of 960 ng·h /mL. Animal/Disease Models: Male ob/ob mice[1] Doses: intravenous (iv) dose, 5 mg/kg; oral dose, 10 mg/kg Route of Administration: intravenous (iv) injection and po (oral gavage); intravenous (iv) dose, 5 mg/kg; oral dose, 10 mg/kg; once Experimental Results: demonstrated a plasma half-life of 1.1 h, a bioavailability of 50%, a Clp of 54 ml/min/kg, an oral Tmax of 0.25 h, an |
| References |
[1]. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experiment. [2]. Design, synthesis, and structure-activity relationships of spirolactones bearing 2-ureidobenzothiophene as acetyl-CoA carboxylases inhibitors. Bioorg Med Chem Lett. 2011 Nov 1;21(21):6314-8. [3]. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One. 2010 Jun 30;5(6):e11394. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.79 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (4.79 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (191.54 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9154 mL | 9.5771 mL | 19.1542 mL | |
| 5 mM | 0.3831 mL | 1.9154 mL | 3.8308 mL | |
| 10 mM | 0.1915 mL | 0.9577 mL | 1.9154 mL |