C646 is a potent, selective and competitive inhibitor for histone acetyltransferase p300. It inhibits p300 with a Ki of 400 nM in a cell-free assay. It is less potent for other acetyltransferases and preferentially selective for p300. C646 induces cell cycle arrest and apoptosis selectively in AML1-ETO-positive AML cells. C646 reverses epithelial to mesenchymal transition of human peritoneal mesothelial cells via blocking TGF-β1/Smad3 signaling pathway in vitro. C646 shows a noncompetitive pattern of p300 inhibition versus H4-15 peptide substrate. C646 treatment reduces histone H3 and H4 acetylation levels and abrogates TSA-induced acetylation in cells.
Physicochemical Properties
| Molecular Formula | C24H19N3O6 | |
| Molecular Weight | 445.42 | |
| Exact Mass | 445.127 | |
| CAS # | 328968-36-1 | |
| Related CAS # |
|
|
| PubChem CID | 1285941 | |
| Appearance | Brown to reddish brown solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 662.6±65.0 °C at 760 mmHg | |
| Melting Point | 224-226℃ | |
| Flash Point | 354.5±34.3 °C | |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C | |
| Index of Refraction | 1.663 | |
| LogP | 4.87 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 7 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 33 | |
| Complexity | 857 | |
| Defined Atom Stereocenter Count | 0 | |
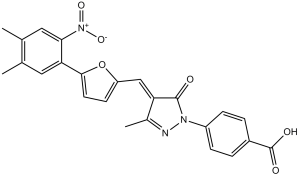
| SMILES | CC1=CC(=C(C=C1C)[N+](=O)[O-])C2=CC=C(O2)/C=C\3/C(=NN(C3=O)C4=CC=C(C=C4)C(=O)O)C |
|
| InChi Key | HEKJYZZSCQBJGB-UNOMPAQXSA-N | |
| InChi Code | InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12H,1-3H3,(H,29,30)/b19-12- | |
| Chemical Name | 4-[(4Z)-4-[[5-(4,5-dimethyl-2-nitrophenyl)furan-2-yl]methylidene]-3-methyl-5-oxopyrazol-1-yl]benzoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | C646 is a linear competitive inhibitor of p300 and acetyl-CoA with Ki of 400 nM. C646 demonstrates a noncompetitive method of p300 inhibition with the H4-15 peptide substrate. C646 treatment lowered histone H3 and H4 acetylation levels and eliminated TSA-induced acetylation in cells. C646 has a more effective effect on cell proliferation than Lys-CoA-Tat [1]. C646 promotes mitotic catastrophe after IR and suppresses the phosphorylation of CHK1 following IRin in A549 cells [2]. C646 attenuates the rise in GATA1 acetylation and the EDAG-induced increase in GATA1 transcriptional activity [3]. |
| ln Vivo | Inhibition of P300 by c646 (intraperitoneal injection, 30 nmol/g/d, for 2 weeks) dramatically lowered blood glucose levels in db/db mice [4]. |
| Animal Protocol |
Animal/Disease Models: Fourteenweeks old male db/db mice and normal m/m mice[4] Doses: 30 nmol/g Route of Administration: Intraperitoneally injected; daily; 2 weeks Experimental Results: The db/db mice demonstrated greater body masses and higher levels of fasting blood glucose than the m/m mice. |
| References |
[1]. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010 May 28;17(5):471-82. [2]. C646, a selective small molecule inhibitor of histone acetyltransferase p300, radiosensitizes lung cancer cells by enhancing mitotic catastrophe. Radiother Oncol. 2014 May;111(2):222-7. [3]. EDAG positively regulates erythroid differentiation and modifies GATA1 acetylation through recruiting p300. Stem Cells. 2014 Aug;32(8):2278-89. [4]. Type 2 diabetes-induced overactivation of P300 contributes to skeletal muscle atrophy by inhibiting autophagic flux. Life Sci. 2020 Aug 10;258:118243. |
| Additional Infomation | C646 is a pyrazolone that is 5-methyl-4-methylene-2-(p-carboxyphenyl)-2,4-dihydro-3H-pyrazol-3-one in which the exocyclic carbon of the methylene group is attached to a 5-(4,5-dimethyl-2-nitrophenyl)furan-2-yl group by a single bond. C646 is a potent, cell permeable and selective competitive inhibitor of p300 and CBP (p300/CBP) histone acetyltransferases. It has a role as an EC 2.3.1.48 (histone acetyltransferase) inhibitor, an apoptosis inducer and a radiosensitizing agent. It is a member of furans, a biaryl, a pyrazolone, a member of benzoic acids and a C-nitro compound. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 1.67 mg/mL (3.75 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 1.67 mg/mL (3.75 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: 5% DMSO+30% PEG 300+ddH2O:1mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2451 mL | 11.2254 mL | 22.4507 mL | |
| 5 mM | 0.4490 mL | 2.2451 mL | 4.4901 mL | |
| 10 mM | 0.2245 mL | 1.1225 mL | 2.2451 mL |