BRD9539 is a potent and selective inhibitor that inhibits G9a activity with an IC50 of 6.3 μM, it also inhibits euchromatin histone methyltransferase 2 (EHMT2). Post-translational modifications of histones alter chromatin structure and play key roles in gene expression and specification of cell states. Small molecules that target chromatin-modifying enzymes selectively are useful as probes and have promise as therapeutics, although very few are currently available. G9a (also named euchromatin histone methyltransferase 2 (EHMT2)) catalyzes methylation of lysine 9 on histone H3 (H3K9), a modification linked to aberrant silencing of tumor-suppressor genes, among others.
Physicochemical Properties
| Molecular Formula | C24H21N3O3 |
| Molecular Weight | 399.4418 |
| Exact Mass | 399.158 |
| CAS # | 1374601-41-8 |
| PubChem CID | 73755260 |
| Appearance | White to off-white solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 676.4±57.0 °C at 760 mmHg |
| Flash Point | 362.9±32.1 °C |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.650 |
| LogP | 5.82 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 30 |
| Complexity | 586 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | WPXMEOBILYVKBC-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C24H21N3O3/c28-22(18-11-5-2-6-12-18)26-24-25-20-16-19(23(29)30)13-14-21(20)27(24)15-7-10-17-8-3-1-4-9-17/h1-6,8-9,11-14,16H,7,10,15H2,(H,29,30)(H,25,26,28) |
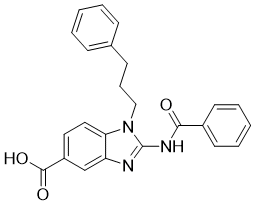
| Chemical Name | 2-benzamido-1-(3-phenylpropyl)benzimidazole-5-carboxylic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | In HeLa cells, BRD9539 lowers ATP levels in a dose-dependent manner [1]. Compared to its methyl ester counterpart BRD4770, BRD9539 is a more powerful biochemical inhibitor, having a residual G9a activity of 20% at screening doses as opposed to 45% for BRD4770. In contrast to BRD4770, BRD9539 did not do well in cell-based experiments, perhaps because of decreased cell permeability. Furthermore, in the presence of 5 or 10 μM BRD9539, the activity of 100 kinases and 16 additional chromatin-modifying enzymes implicated in cancer cell biology and cell cycle regulation was examined; no activity was detected in these assays [1]. |
| References |
[1]. A small-molecule probe of the histone methyltransferase G9a induces cellular senescence in pancreatic adenocarcinoma. ACS Chem Biol. 2012 Jul 20;7(7):1152-7. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~16.67 mg/mL (~41.73 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 1.67 mg/mL (4.18 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.67 mg/mL (4.18 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 16.7 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5035 mL | 12.5175 mL | 25.0350 mL | |
| 5 mM | 0.5007 mL | 2.5035 mL | 5.0070 mL | |
| 10 mM | 0.2504 mL | 1.2518 mL | 2.5035 mL |