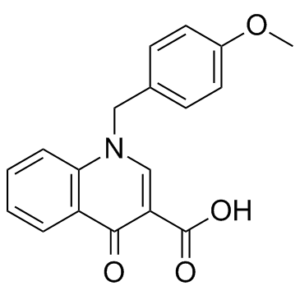
BQCA, a benzylquinolone carboxylic acid, is a potent, highly selective positive allosteric modulator of the M1 muscarinic acetylcholine receptor (mAChR). M(1) muscarinic acetylcholine receptors (mAChRs) could be a promising target for the treatment of conditions involving reduced cognitive function. The absence of highly selective ligands for specific mAChR subtypes has, however, significantly hampered the testing of this theory. By acting at an allosteric site, BQCA increases functional responses to orthosteric agonists without directly activating the receptor. Research on radioligand binding has demonstrated that BQCA raises the acetylcholine affinity of the M(1) receptor. Acute slices from M(1) receptor knock-out mice lack the strong inward current and increased spontaneous EPSCs that are induced when the M(1) receptor is activated by BQCA in medial prefrontal cortex (mPFC) pyramidal cells.
Physicochemical Properties
| Molecular Formula | C18H15NO4 | |
| Molecular Weight | 309.32 | |
| Exact Mass | 309.1 | |
| Elemental Analysis | C, 69.89; H, 4.89; N, 4.53; O, 20.69 | |
| CAS # | 338747-41-4 | |
| Related CAS # |
|
|
| PubChem CID | 1476756 | |
| Appearance | White to off-white solid powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 492.6±45.0 °C at 760 mmHg | |
| Flash Point | 251.7±28.7 °C | |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C | |
| Index of Refraction | 1.649 | |
| LogP | 2.83 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 23 | |
| Complexity | 493 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O=C1C(C(=O)O[H])=C([H])N(C2=C([H])C([H])=C([H])C([H])=C21)C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])[H] |
|
| InChi Key | BZBBTGCKPRSPGF-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C18H15NO4/c1-23-13-8-6-12(7-9-13)10-19-11-15(18(21)22)17(20)14-4-2-3-5-16(14)19/h2-9,11H,10H2,1H3,(H,21,22) | |
| Chemical Name | 1-[(4-methoxyphenyl)methyl]-4-oxoquinoline-3-carboxylic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | BQCA can 129-fold lower the concentration of ACh needed to activate M1 with an inflection point value of 845 nM. Up to 100 μM, there is no evidence of agonism, antagonism, or potentiation on other mAChRs[1]. BQCA raises acetylcholine affinity at the M1 receptor. In medial prefrontal cortex (mPFC) pyramidal cells, BQCA-induced M1 receptor activation results in a strong inward current and an increase in spontaneous excitatory postsynaptic currents[2]. |
| ln Vivo | BQCA necessary for M1 to enhance c-fos and arc RNA expression, ERK phosphorylation, and inositol phosphate turnover in primary neurons. BQCA increases cerebral cortex blood flow, increases wakefulness while decreasing delta sleep, and reverses the memory deficits caused by scopolamine in contextual fear conditioning. A role for this signal transduction mechanism in the cholinergic modulation of memory is suggested by BQCA's induction of β-arrestin recruitment to M1[1]. In vivo, BQCA promotes mPFC pyramidal cell firing. In a transgenic mouse model of Alzheimer's disease, BQCA also reinstates discrimination reversal learning[2]. |
| Enzyme Assay | In 96-well deep-well plates, competition binding reactions were conducted using 25 μg human M1 CHO membrane protein, BQCA or vehicle, and 0.15 nM [3H]NMS. Fast filtration stops binding reactions that take place at 30 °C for two to three hours. To measure nonspecific binding, 10 μM atropine is added. Ice-cold filter plates are ished 4x. using a 96-well harvester, 20 mM HEPES, 100 mM NaCl, and 5 mM MgCl2, pH 7.4. After drying the plates, a microplate scintillation counter is used to count the radioactivity on them[1]. |
| Animal Protocol |
Rats: Male Sprague-Dawley rats weighing between 225 and 250 g are given an intraperitoneal injection (10 mg/kg) of a BQCA microsuspension containing 10% tween 80. Samples of whole brain tissue and blood are taken at 0.5, 1, 2, 4, and 8 hours. Using a cardiac puncture, blood samples are drawn into EDTA vacutainer tubes. After centrifugation, the plasma is separated and kept at -80°C until analysis. The animals are beheaded, and their entire brain tissue is extracted and frozen on dry ice right away[2]. Mice: 30 minutes before being placed in a chamber for 2 minutes and subjected to 2 tone-footshock pairings (3 kHz, 85 dB tone for 30 s co-terminated with a 0.5 mA, 1 s shock) 2 minutes apart, mice are given an intraperitoneal injection of BQCA in 5% beta-cyclodextrin and/or 0.3 mg/kg scopolamine in 0.9% saline. After 30 seconds of the last pairing, the mice are returned to their home cage. Mice are placed in the same chamber 24 hours later, and Video Freeze is used to measure the freezing point[1]. |
| References |
[1]. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15950-5. [2]. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptorincreases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009 Nov 11;29(45):14271-86. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2329 mL | 16.1645 mL | 32.3290 mL | |
| 5 mM | 0.6466 mL | 3.2329 mL | 6.4658 mL | |
| 10 mM | 0.3233 mL | 1.6164 mL | 3.2329 mL |