|
BMY 7378 (BMY-7378; BMY7378) dihydrochloride is a multi-target compound that inhibits α2C-adrenoceptor and α1D-adrenoceptor with pKi values of 6.54 and 8.2, respectively.
|
Physicochemical Properties
| Molecular Formula |
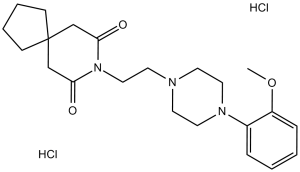
C22H33CL2N3O3
|
| Molecular Weight |
458.42
|
| Exact Mass |
457.189
|
| Elemental Analysis |
C, 57.64; H, 7.26; Cl, 15.47; N, 9.17; O, 10.47
|
| CAS # |
21102-95-4
|
| Related CAS # |
21102-94-3 (Free base); 21102-95-4 (HCl)
|
| PubChem CID |
210172
|
| Appearance |
Typically exists as White to off-white solid at room temperature
|
| Boiling Point |
585.6ºC at 760 mmHg
|
| Melting Point |
196.5-198.5 °C
|
| Flash Point |
307.9ºC
|
| Vapour Pressure |
1.07E-13mmHg at 25°C
|
| LogP |
4.071
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
30
|
| Complexity |
547
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
Cl[H].Cl[H].O=C1C([H])([H])C2(C([H])([H])C(N1C([H])([H])C([H])([H])N1C([H])([H])C([H])([H])N(C3=C([H])C([H])=C([H])C([H])=C3OC([H])([H])[H])C([H])([H])C1([H])[H])=O)C([H])([H])C([H])([H])C([H])([H])C2([H])[H]
|
| InChi Key |
NIBOMXUDFLRHRV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H31N3O3.2ClH/c1-28-19-7-3-2-6-18(19)24-13-10-23(11-14-24)12-15-25-20(26)16-22(17-21(25)27)8-4-5-9-22;;/h2-3,6-7H,4-5,8-17H2,1H3;2*1H
|
| Chemical Name |
8-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-8-azaspiro[4.5]decane-7,9-dione;dihydrochloride
|
| Synonyms |
| BMY 7378; BMY-7378; 21102-95-4; BMY 7378 dihydrochloride; BMY 7378; 8-(2-(4-(2-Methoxyphenyl)piperazin-1-yl)ethyl)-8-azaspiro[4.5]decane-7,9-dione dihydrochloride; BMY7378; 8-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-8-azaspiro[4.5]decane-7,9-dione;dihydrochloride; KC07KV8T5O; 1,1-Cyclopentanediacetimide, N-(2-(4-(o-methoxyphenyl)-1-piperazinyl)ethyl)-, dihydrochloride; BMY7378 |
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder-20°C 3 years
4°C 2 years
In solvent -80°C 6 months
-20°C 1 month
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
|
Biological Activity
| Targets |
5-HT1A ( pIC50 = 8.3 ); α1D-adrenoceptor ( pKi = 8.2 ); Dopamine D2 receptor ( pIC50 = 7.4 ); α2C-adrenoceptor ( pKi = 6.54 ); 5-HT1C ( pIC50 = 6.4 )
|
| ln Vitro |
In vitro activity: BMY 7378 exhibits selectivity for the alpha 1D-adrenoceptor subtype (pKi: hamster alpha 1b-adrenoceptor 6.2, human alpha 1b-adrenoceptor 7.2; bovine alpha 1c-adrenoceptor 6.1, human alpha 1c-adrenoceptor 6.6; rat alpha 1d-adrenoceptor 8.2, human alpha 1d-adrenoceptor 9.4) and exhibits a high affinity (pA2, 8.9) for rat aorta alpha 1-adrenoceptor][2]. |
|
| ln Vivo |
| BMY 7378 (pA2 of 8.67) is approximately 100 times more potent than yohimbine (pA2 of 6.62) against contractions to noradrenaline in rat aorta. BMY 7378 (pA2 of 6.48) is approximately 10 times less potent than yohimbine (pA2 of 7.56) at antagonizing the contractile response to noradrenaline in human saphenous vein (α2C-adrenoceptor). BMY 7378 dose dependently (0.25-5 mg/kg s.c.) reduces the undetectable levels forepaw treading and head weaving induced by 8-OH-DPAT (0.75 mg/kg s.c.) in rats. BMY 7378 causes a marked and dose-dependent (0.01-1.0 mg/kg s.c.) decrease of 5-HT release in ventral hippocampus of the anaesthetized rat as detected by brain microdialysis in rats. |
|
| Enzyme Assay |
BMY 7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8- azaspiro[4.5]decane-7,9-dione dihydrochloride), a 5-HT1A receptor partial agonist, also binds to alpha 1-adrenoceptors. Competition assays were performed using (+/-)-beta-([125I]iodo-4-hydroxyphenyl)-ethyl-aminomethyl-tetralone ([125I]HEAT), and membranes prepared from Rat-1 fibroblasts expressing hamster alpha 1b-, bovine alpha 1c-, or rat alpha 1d-adrenoceptor, or their respective human homologues. Results indicate that BMY 7378 is selective for the alpha 1D-adrenoceptor subtype (pKi: hamster alpha 1b-adrenoceptor 6.2 +/- 0.03, human alpha 1b-adrenoceptor 7.2 +/- 0.05; bovine alpha 1c-adrenoceptor 6.1 +/- 0.02, human alpha 1c-adrenoceptor 6.6 +/- 0.20; rat alpha 1d-adrenoceptor 8.2 +/- 0.06, human alpha 1d-adrenoceptor 9.4 +/- 0.05) and has high affinity (pA2, 8.9 +/- 0.1) for rat aorta alpha 1-adrenoceptor. [2]
|
| Cell Assay |
With a selective antagonist, the specific contribution of the alpha-1D adrenoceptor (AR) to vascular smooth muscle contraction has been assessed. BMY 7378 bound to membranes expressing the cloned rat alpha-1D AR with a > 100-fold higher affinity (K1 = 2 nM) than binding to either the cloned rat alpha-1A AR (Ki = 800 nM) or the hamster alpha-1B AR (Ki = 600 nM). BMY 7378 exhibited differential potency in inhibiting vascular smooth muscle contraction. In the rat aorta and iliac artery, BMY 7378 was a high-affinity antagonist, producing parallel shifts in the phenylephrine concentration-response curve. The dissociation constants for this compound by Schild analysis were 0.95 and 4 nM for the aorta and iliac artery, respectively. The slopes of these Schild plots were not significantly different from unity. BMY 7378 was a weak antagonist in the rat caudal, mesenteric resistance and renal arteries, with Schild slopes significantly < 1. With ribonuclease protection assays, alpha-1D mRNA was found in all blood vessels examined. These data suggest that (1) BMY 7378 is a selective alpha-1D AR antagonist that can be used in functional systems to assess the contribution of this receptor in vascular smooth muscle contraction; (2) the alpha-1D AR appears to play a major role in the contraction of the aorta and iliac artery; (3) despite the fact that the mRNA for the alpha-1D AR can be detected in the caudal, mesenteric resistance (4) and renal arteries, it does not appear to play a role in mediating contraction of these blood vessels; and (4) expression of alpha-1D mRNA in a particular artery does not ensure that this receptor is involved in regulating the contraction of that artery. [1]
|
| Animal Protocol |
| Dissolved in 0.9% sodium chloride solution; 0.25-5 mg/kg; s.c. injection | | Rat | |
|
| Toxicity/Toxicokinetics |
mouse LD50 intraperitoneal 53600 ug/kg Journal of Medicinal Chemistry., 12(876), 1969
|
| References |
[1]. The specific contribution of the novel alpha-1D adrenoceptor to the contraction of vascular smooth muscle. J Pharmacol Exp Ther. 1995;275(3):1583-1589.
[2]. BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1995;272(2-3):R5-R6.
|
| Additional Infomation |
Adrenergic alpha-Antagonists:
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma.
|
|
Solubility Data
| Solubility (In Vitro) |
| DMSO: ~92 mg/mL (~200.7 mM) | | Water: ~92 mg/mL (~200.7 mM) | | Ethanol: ~20 mg/mL (~43.6 mM) |
|
| Solubility (In Vivo) |
(Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions |
|
1 mg |
5 mg |
10 mg |
| 1 mM |
2.1814 mL |
10.9070 mL |
21.8141 mL |
| 5 mM |
0.4363 mL |
2.1814 mL |
4.3628 mL |
| 10 mM |
0.2181 mL |
1.0907 mL |
2.1814 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles. |